MORPHINE SULFATE injection
Morphine Sulfate by
Drug Labeling and Warnings
Morphine Sulfate by is a Prescription medication manufactured, distributed, or labeled by Civica, Inc., Hikma Pharmaceuticals USA Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use morphine sulfate injection, USP safely and effectively. See full prescribing information for morphine sulfate injection, USP.
MORPHINE sulfate injection, USP, preservative-free, solution for intravenous use, CII
Initial U.S. Approval: 1984INDICATIONS AND USAGE
Morphine sulfate is an opioid agonist indicated for the management of pain not responsive to non-narcotic analgesics. (1)
DOSAGE AND ADMINISTRATION
- Direct Intravenous Injection: The usual starting dose in adults is 0.1 mg to 0.2 mg per kg every 4 hours as needed for pain management. The dose should be adjusted according to the severity of pain, the occurrence of adverse events, as well as the patient’s underlying disease, age, and size. (2.2, 2.3)
DOSAGE FORMS AND STRENGTHS
- Morphine sulfate injection, 4 mg/mL is available in 1 mL single dose vials for intravenous administration. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Dosing errors: Take care when prescribing and administering to avoid dosing errors due to confusion between different concentrations and between mg and mL, which could result in accidental overdose and death. (5.1)
- Cardiovascular instability: High doses are excitatory, resulting from sympathetic hyperactivity and increase in circulatory catecholamine (5.2)
- Respiratory depression: Rapid intravenous administration may result in chest wall rigidity (5.3)
- CNS toxicity: High doses are excitatory, resulting in convulsions (5.4)
- CNS Depressants: May increase the risk of respiratory depression, hypotension, sedation, coma, or death if used in conjunction with other CNS active drugs (5.6)
- Increased intracranial pressure or head injury: May increase respiratory depressant effects and elevate cerebrospinal fluid pressure (5.7)
- Hypotensive effect: May cause hypotension in ambulatory patients (5.8)
- Gastrointestinal effects: May diminish propulsive peristaltic waves in the gastrointestinal tract and prolong obstruction (5.10)
- Biliary surgery or disorders of biliary tract: May cause spasm of the sphincter of Oddi and diminish biliary and pancreatic secretions (5.11)
ADVERSE REACTIONS
The most serious adverse reactions encountered are respiratory depression, apnea, circulatory depression, respiratory arrest, shock, and cardiac arrest. Other common frequently observed adverse reactions include: sedation, lightheadedness, dizziness, nausea, vomiting, and constipation. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- CNS depressants: May increase the risk of respiratory depression (7.1)
- Muscle relaxants: May enhance the neuromuscular blocking action of skeletal muscle relaxants and produce respiratory depression (7.2)
- Mixed agonist/antagonist opioid analgesics: May reduce the analgesic effect and/or may precipitate withdrawal symptoms (7.3)
- Cimetidine: May increase respiratory and CNS depression (7.4)
- Anticholinergics: May increase the risk of urinary retention, severe constipation, or paralytic ileus (7.6)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on animal data, may cause fetal harm (8.1)
- Pediatric patients: Safety and effectiveness and the pharmacokinetics of morphine sulfate injection in pediatric patients below the age of 18 have not been established. (8.4)
- Geriatric patients: Use caution during dose selection, starting at the low end of the dosing range while carefully monitoring for side effects. (8.5)
- Renal and hepatic impairment: Start patients at lower doses and titrate cautiously (8.7, 8.8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Considerations
2.2 Individualization of Dosage
2.3 Direct Intravenous Injection
2.4 Dosing with Hepatic and Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Medication Errors
5.2 Cardiovascular Instability
5.3 Respiratory Depression
5.4 Central Nervous System (CNS) Toxicity
5.5 Misuse, Abuse and Diversion of Opioids
5.6 Central Nervous System (CNS) Depressants
5.7 Increased Intracranial Pressure or Head Injury
5.8 Hypotensive Effect
5.9 Driving and Operating Machinery
5.10 Gastrointestinal Effects
5.11 Use in Biliary Surgery or Disorders of the Biliary Tract
5.12 Special Risk Groups
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Central Nervous System (CNS) Depressants
7.2 Muscle Relaxants
7.3 Mixed Agonist/Antagonist Opioid Analgesics
7.4 Cimetidine
7.5 Monoamine Oxidase Inhibitors (MAOIs)
7.6 Anticholinergics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Gender
8.7 Hepatic Impairment
8.8 Renal Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
10.1 Symptoms
10.2 Treatment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Morphine sulfate injection is intended for intravenous administration.
2.1 General Dosing Considerations
Morphine sulfate injection is available in three concentrations for direct injection. Take care when prescribing and administering morphine sulfate injection to avoid dosing errors due to confusion between different concentrations and between mg and mL, which could result in accidental overdose and death. Take care to ensure the proper dose is communicated and dispensed. When writing prescriptions, include both the total dose in mg and total dose in volume.
Administration of morphine sulfate injection should be limited to use by those familiar with the management of respiratory depression. Morphine must be injected slowly; rapid intravenous administration may result in chest wall rigidity.
Selection of patients for treatment with morphine sulfate injection should be governed by the same principles that apply to the use of similar opioid analgesics. Individualize treatment in every case, using non-opioid analgesics, opioids on an as needed basis and/or combination products, and chronic opioid therapy in a progressive plan of pain management such as outlined by the World Health Organization, the Agency for Healthcare Research and Quality, and the American Pain Society.
2.2 Individualization of Dosage
Adjust the dosing regimen for each patient individually, taking into account the patient’s prior analgesic treatment experience. In the selection of the initial dose of morphine sulfate injection, give attention to the following:
- the total daily dose, potency and specific characteristics of the opioid the patient has been taking previously;
- the reliability of the relative potency estimate used to calculate the equivalent morphine sulfate injection dose needed;
- the patient’s degree of opioid tolerance;
- the general condition and medical status of the patient;
- concurrent medications;
- the type and severity of the patient’s pain;
- risk factors for abuse, addiction or diversion, including prior history of abuse, addiction or diversion
The following dosing recommendations, therefore, can only be considered suggested approaches to what is actually a series of clinical decisions over time in the management of the pain of each individual patient.
Continual reevaluation of the patient receiving morphine sulfate injection is important, with special attention to the management of pain and the relative incidence of side effects associated with therapy.
During periods of changing analgesic requirements, including initial titration, frequent contact is recommended between the physician, other members of the healthcare team, the patient, and the caregiver/family.
2.3 Direct Intravenous Injection
The usual starting dose in adults is 0.1 mg to 0.2 mg per kg every 4 hours as needed to manage pain. Administer the injection slowly.
2.4 Dosing with Hepatic and Renal Impairment
Morphine sulfate pharmacokinetics have been reported to be significantly altered in patients with cirrhosis and renal failure. Start these patients cautiously with lower doses of morphine sulfate injection and titrate slowly while carefully monitoring for side effects. [See Use in Specific Populations (8.7 and 8.8).]
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- Morphine sulfate is contraindicated in patients with known hypersensitivity to morphine.
- Morphine sulfate is contraindicated in patients with respiratory depression in the absence of resuscitative equipment.
- Morphine sulfate is contraindicated in patients with acute or severe bronchial asthma or hypercarbia.
- Morphine sulfate is contraindicated in any patient who has or is suspected of having a paralytic ileus.
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Medication Errors
Morphine sulfate injection is available in three concentrations for direct injection. Take care when prescribing and administering morphine sulfate injection to avoid dosing errors due to confusion between different concentrations and between mg and mL, which could result in accidental overdose and death. Take care to ensure the proper dose is communicated and dispensed. When writing prescriptions, include both the total dose in mg and the total dose in volume.
5.2 Cardiovascular Instability
While low doses of intravenously administered morphine have little effect on cardiovascular stability, high doses are excitatory, resulting from sympathetic hyperactivity and increase in circulatory catecholamines. Have naloxone injection and resuscitative equipment immediately available for use in case of life-threatening or intolerable side effects and whenever morphine therapy is being initiated.
5.3 Respiratory Depression
Respiratory depression is the primary risk of morphine sulfate injection. Respiratory depression occurs more frequently in elderly or debilitated patients and in those suffering from conditions accompanied by hypoxia, hypercapnia, or upper airway obstruction, in whom even moderate therapeutic doses may significantly decrease pulmonary ventilation. Morphine administration should be limited to use by those familiar with the management of respiratory depression. Rapid intravenous administration may result in chest wall rigidity.
Patients with chronic obstructive pulmonary disease or cor pulmonale and in patients having a substantially decreased respiratory reserve (e.g., severe kyphoscoliosis), hypoxia, hypercapnia, or preexisting respiratory depression have an increased risk of increased airway resistance and decrease respiratory drive to the point of apnea with use of morphine sulfate injection. Therefore, consider alternative non-opioid analgesics, and use morphine sulfate injection only under careful medical supervision at the lowest effective dose in such patients.
5.4 Central Nervous System (CNS) Toxicity
Excitation of the central nervous system, resulting in convulsion, may accompany high doses of morphine given intravenously. Dysphoric reactions may occur after any size dose and toxic psychoses have been reported.
5.5 Misuse, Abuse and Diversion of Opioids
Morphine sulfate is an opioid agonist and Schedule II controlled substance. Such drugs are sought by drug abusers and people with addiction disorders. Diversion of Schedule II products is an act subject to criminal penalty. [See Drug Abuse and Dependence (9).]
Morphine sulfate can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing morphine sulfate injection in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse, or diversion.
Concerns about abuse, addiction, and diversion should not prevent the proper management of pain. Healthcare professionals should contact their State of Professional Licensing Board or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
5.6 Central Nervous System (CNS) Depressants
The depressant effects of morphine are potentiated by the presence of other CNS depressants such as alcohol, sedatives, antihistamines, or psychotropic drugs. Use of morphine in conjunction with other CNS active drugs may increase the risk of respiratory depression, hypotension, profound sedation, coma, or death.
5.7 Increased Intracranial Pressure or Head Injury
Use morphine sulfate injection with extreme caution in patients with head injury or increased intracranial pressure. In the presence of head injury, intracranial lesions or a preexisting increase in intracranial pressure, the possible respiratory depressant effects of morphine sulfate injection and its potential to elevate cerebrospinal fluid pressure (resulting from vasodilation following CO2 retention) may be markedly exaggerated. Pupillary changes (miosis) from morphine may obscure the existence, extent and course of intracranial pathology. Clinicians should maintain a high index of suspicion for adverse drug reactions when evaluating altered mental status or movement abnormalities in patients receiving this modality of treatment.
5.8 Hypotensive Effect
Morphine sulfate injection may cause severe hypotension in an individual whose ability to maintain their blood pressure has been compromised by depleted blood volume, shock, impaired myocardial function or concurrent administration of sympatholytic drugs, and drugs such as phenothiazines or general anesthetics. Orthostatic hypotension is a frequent complication in single-dose parenteral morphine analgesia in ambulatory patients.
The vasodilation produced by morphine sulfate injection may further reduce cardiac output and blood pressure in patients in circulatory shock.
5.9 Driving and Operating Machinery
Morphine may impair the mental and physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Caution patients accordingly.
5.10 Gastrointestinal Effects
Do not administer morphine sulfate injection to patients with gastrointestinal obstruction, especially paralytic ileus because morphine sulfate injection diminishes propulsive peristaltic waves in the gastrointestinal tract and may prolong the obstruction.
The administration of morphine sulfate injection may obscure the diagnosis or clinical course in patients with acute abdominal condition.
5.11 Use in Biliary Surgery or Disorders of the Biliary Tract
Morphine sulfate injection may cause spasm of the sphincter of Oddi and diminish biliary and pancreatic secretions.
5.12 Special Risk Groups
Use morphine sulfate injection with caution and in reduced dosages in patients with severe renal or hepatic impairment, Addison’s disease, hypothyroidism, prostatic hypertrophy, or urethral stricture, and in elderly or debilitated patients. [See Use in Specific Populations (8.5).]
Exercise caution in the administration of morphine sulfate injection to patients with CNS depression, toxic psychosis, acute alcoholism and delirium tremens.
-
6 ADVERSE REACTIONS
Serious adverse reactions associated with morphine sulfate injection include, respiratory depression, apnea, and to a lesser degree, circulatory depression, respiratory arrest, shock, and cardiac arrest. Rarely, anaphylactoid reactions have been reported when morphine or other phenanthrene alkaloids of opium are administered intravenously.
The most frequently observed adverse reactions include sedation, lightheadedness, dizziness, nausea, vomiting, constipation, and diaphoresis. These effects seem to be more prominent in ambulatory patients and in those who are not experiencing severe pain. Some adverse reactions in ambulatory patients may be alleviated if the patient lies down.
Other possible adverse reactions include:
Central Nervous System – Euphoria, dysphoria, weakness, headache, agitation, tremor, uncoordinated muscle movements, visual disturbances, transient hallucinations and disorientation.
Gastrointestinal – Constipation, biliary tract spasm.
Cardiovascular – Tachycardia, bradycardia, palpitation, faintness, syncope, and orthostatic hypotension.
Genitourinary – Oliguria and urinary retention; an antidiuretic effect has been reported.
Allergic – Pruritus, urticaria, and skin rashes. Anaphylactoid reactions have been reported following intravenous administration.
Other – Opiate-induced histamine release may be responsible for the flushing of the face, diaphoresis, and pruritus often seen with these drugs. Wheals and urticaria at the site of injection are probably related to histamine release. Local tissue irritation, pain and induration have been reported following repeated subcutaneous injection. Morphine may alter temperature regulation in susceptible individuals and will depress the cough reflex. -
7 DRUG INTERACTIONS
7.1 Central Nervous System (CNS) Depressants
Morphine should be administered cautiously to avoid additive effects when other central nervous system depressants, including other narcotic analgesics, general anesthetics, phenothiazines, tricyclic antidepressants, tranquilizers, sedatives, hypnotics, antiemetics, and alcohol are given concomitantly. When given concomitantly the risks of respiratory depression, hypotension, profound sedation and coma are increased.
7.2 Muscle Relaxants
Morphine sulfate may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression.
7.3 Mixed Agonist/Antagonist Opioid Analgesics
Do not administer mixed agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, and butorphanol) to patients who have received or are receiving a course of therapy with a pure opioid agonist analgesic such as morphine sulfate injection. In these patients, mixed agonist/antagonist analgesics may reduce the analgesic effect and/or may precipitate withdrawal symptoms.
7.4 Cimetidine
Concomitant administration of morphine sulfate injection and cimetidine has been reported to precipitate apnea, confusion, and muscle twitching in an isolated report. Monitor patients for increased respiratory and CNS depression when receiving cimetidine concomitantly with morphine sulfate injection.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects (Pregnancy Category C)
No formal studies to assess the teratogenic effects of morphine in animals have been conducted. It is also not known whether morphine can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Morphine should be given to a pregnant woman only if clearly needed.
In humans, the frequency of congenital anomalies have been reported to be no greater than expected among the children of 70 women who were treated with morphine during the first four months of pregnancy or in 448 women treated with morphine anytime during pregnancy. Furthermore, no malformations were observed in the infant of a woman who attempted suicide by taking an overdose of morphine and other medication during the first trimester of pregnancy.
Several literature reports indicate that morphine administered subcutaneously during the early gestational period in mice and hamsters produced neurological, soft tissue and skeletal abnormalities. With one exception, the effects that have been reported were following doses that were maternally toxic and the abnormalities noted were characteristic of those observed when maternal toxicity is present.
In one study, following subcutaneous infusion of doses greater than or equal to 0.15 mg/kg to mice, exencephaly, hydronephrosis, intestinal hemorrhage, split subpraoccipital, malformed sternebrae, and malformed xiphoid were noted in the absence of maternal toxicity. In the hamster, morphine sulfate given subcutaneously on gestation day 8 produced exencephaly and cranioschisis. In rats treated with subcutaneous infusions of morphine during the period of organogenesis, no teratogenicity was observed. No maternal toxicity was observed in this study, however, increased mortality and growth retardation were seen in the offspring. In two studies performed in the rabbit, no evidence of teratogenicity was reported at subcutaneous doses up to 100 mg/kg.Nonteratogenic Effects
Controlled studies of chronic in utero morphine exposure in pregnant women have not been conducted. Infants born to mothers who have taken opioids chronically may exhibit withdrawal symptoms, reversible reduction in brain volume, small size, decreased ventilatory response to CO2 and increased risk of sudden infant death syndrome. Morphine sulfate should be used by a pregnant woman only if the need for opioid analgesia clearly outweighs the potential risks to the fetus.
Published literature has reported that exposure to morphine during pregnancy is associated with reduction in growth and a host of behavioral abnormalities in the offspring of animals. Morphine treatment during gestational periods of organogenesis in rats, hamsters, guinea pigs and rabbits resulted in the following treatment-related embryotoxicity and neonatal toxicity in one or more studies: decreased litter size, embryo-fetal viability, fetal and neonatal body weights, absolute brain and cerebellar weights, delayed motor and sexual maturation, and increased neonatal mortality, cyanosis and hypothermia. Decreased fertility in female offspring, and decreased plasma and testicular levels of luteinizing hormone and testosterone, decreased testes weights, seminiferous tubule shrinkage, germinal cell aplasia, and decreased spermatogenesis in male offspring were also observed. Decreased litter size and viability were observed in the offspring of male rats administered morphine (25 mg/kg, ip) for 1 day prior to mating. Behavioral abnormalities resulting from chronic morphine exposure of fetal animals included altered reflex and motor skill development, mild withdrawal, and altered responsiveness to morphine persisting into adulthood.
8.2 Labor and Delivery
Morphine readily passes into the fetal circulation and may result in respiratory depression and psycho-physiologic effects in neonates. Naloxone and resuscitative equipment should be available for reversal of narcotic-induced respiratory depression in the neonate. In addition, parenteral morphine may reduce the strength, duration and frequency of uterine contractions resulting in prolonged labor. However, this effect is not consistent and may be offset by an increased rate of cervical dilatation, which tends to shorten labor. Closely observe neonates whose mothers received opioid analgesics during labor for signs of respiratory depression.
8.3 Nursing Mothers
Low levels of morphine sulfate injection have been detected in maternal milk. The milk:plasma morphine AUC ratio is about 2:5:1. The amount of morphine sulfate injection delivered to the infant depends on the plasma concentration of the mother, the amount of milk ingested by the infant, and the extent of first-pass metabolism. Because of the potential for serious adverse reactions in nursing infants from morphine sulfate injection including respiratory depression, sedation and possibly withdrawal symptoms, upon cessation of morphine sulfate injection administration to the mother, decide whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of morphine sulfate injection in pediatric patients below the age of 18 have not been established.
8.5 Geriatric Use
The pharmacodynamic effects of morphine in the elderly are more variable than in the younger population. Older patients will vary widely in the effective initial dose, rate of development of tolerance and the frequency and magnitude of associated adverse effects as the dose is increased. Initial doses should be based on careful clinical observation following “test doses”, after making due allowances for the effects of the patient’s age and infirmity on his/her ability to clear the drug.
Elderly patients may be more susceptible to respiratory depression and/or respiratory arrest following administration of morphine.
In general, use caution when selecting a dose for an elderly patient, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
8.6 Gender
While evidence of greater post-operative morphine sulfate injection consumption in men compared to women is present in the literature, clinically significant differences in analgesic outcomes and pharmacokinetic parameters have not been consistently demonstrated. Some studies have shown an increased sensitivity to the adverse effects of morphine sulfate injection, including respiratory depression, in women compared to men.
8.7 Hepatic Impairment
Morphine sulfate pharmacokinetics have been reported to be significantly altered in patients with cirrhosis. Clearance was found to decrease with a corresponding increase in half-life. The M3G and M6G to morphine AUC ratios also decreased in these subjects, indicating diminished metabolic activity. Start these patients cautiously with lower doses of morphine sulfate injection and titrate slowly while carefully monitoring for side effects.
8.8 Renal Impairment
Morphine sulfate pharmacokinetics are altered in patients with renal failure. Clearance is decreased and the metabolites, M3G and M6G, may accumulate to much higher plasma levels in patients with renal failure as compared to patients with normal renal function. Start these patients cautiously with lower doses of morphine sulfate injection and titrate slowly while carefully monitoring for side effects.
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Morphine sulfate is a mu-opioid agonist and a Schedule II controlled substance. Morphine sulfate, like other opioids, can be abused and is subject to criminal diversion.
9.2 Abuse
Morphine sulfate injection contains a potent narcotic which has been associated with abuse and dependence. Abuse is defined as the intentional non-therapeutic use of a drug, even once, for its rewarding psychological or physiological effects. Due to the risk of overdosage and the risk of its diversion and abuse, it is recommended that special measures be taken to control this product within the hospital or clinic.
Morphine sulfate injection should be subject to rigid accounting, rigorous control of wastage and restricted access.
“Drug-seeking” behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated “loss” of prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor shopping” to obtain additional prescriptions is common among drug abusers and people suffering from untreated addiction.
Drug addiction is characterized by compulsive use, use for non-medical purposes, and continued use despite harm or risk of harm. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence. The converse is also true. In addition, abuse of opioids can occur in the absence of true addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Careful recordkeeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic reevaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Infants born to mothers physically dependent on opioids will also be physically dependent and may exhibit respiratory difficulties and withdrawal symptoms. [See Use in Specific Populations (8.2).]
9.3 Dependence
Physical dependence is manifested by withdrawal symptoms after abrupt discontinuation of a drug or upon administration of an antagonist. Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors) and euphoria. Physical dependence and tolerance are frequent during chronic opioid therapy.
The opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
Withdrawal symptoms may occur when morphine is discontinued abruptly or upon administration of a narcotic antagonist. In general, taper morphine rather than abruptly discontinue, especially when used for more than a few days.
-
10 OVERDOSAGE
10.1 Symptoms
Acute overdosage of morphine is characterized by respiratory depression, with or without concomitant CNS depression. In severe overdosage, apnea, circulatory collapse, cardiac arrest and death may occur.
Morphine sulfate may cause miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdosage but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origin may produce similar findings). Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations.
10.2 Treatment
Give primary attention to the reestablishment of adequate respiratory exchange through provision of a patent airway and institution of assisted, or controlled, ventilation. Employ supportive measures (including oxygen and vasopressors) in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
The pure opioid antagonist naloxone is a specific antidote to respiratory depression resulting from opioid overdose. Since the duration of reversal is expected to be less than the duration of action of morphine sulfate injection, carefully monitor the patient until spontaneous respiration is reliably reestablished. If the response to opioid antagonists is sub-optimal or only brief in nature, administer additional antagonist as directed by the manufacturer of the product.
Do not administer opioid antagonists in the absence of clinically significant respiratory or circulatory depression secondary to morphine sulfate injection overdose. Administer such agents cautiously to persons who are known, or suspected to be physically dependent on morphine sulfate injection. In such cases, an abrupt or complete reversal of opioid effects may precipitate an acute abstinence syndrome.
In an individual physically dependent on opioids, administration of the usual dose of the antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal symptoms experienced will depend on the degree of physical dependence and the dose of the antagonist administered. Reserve use of an opioid antagonist for cases where such treatment is clearly needed. If it is necessary to treat serious respiratory depression in the physically dependent patient, initiate administration of the antagonist with care and titrate with smaller than usual doses.
-
11 DESCRIPTION
Morphine is a phenanthrene-derivative opiate agonist. It is the principal alkaloid of opium and is considered to be the prototype of the opiate agonists.
Morphine sulfate occurs as white, feathery, silky crystals; cubical masses of crystals; or a white, crystalline powder.
When exposed to air it gradually loses water of hydration, and darkens on prolonged exposure to light. It is soluble in water and ethanol at room temperature. The chemical name of morphine sulfate is 7,8-Didehydro-4,5-epoxy-17-methyl-(5α,6α)-morphinan-3,6-diol sulfate (2:1) (salt), pentahydrate, with the following structural formula:
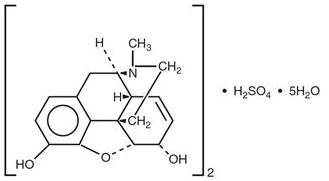
(C17H19NO3)2 H2SO4 5H2O Molecular Weight is 758.83
Morphine sulfate injection, USP is a sterile, nonpyrogenic solution, free of antioxidants and preservatives, intended for intravenous administration. Each milliliter of sterile solution contains 4 mg morphine sulfate and the following inactive ingredients: 0.2 mg edetate disodium, 0.4 mg citric acid monohydrate, sodium chloride to adjust isotonicity and water for injection. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH. The pH range is 2.5 to 4.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Morphine, a full opioid agonist, is relatively selective for the mu receptor, although it can interact with other opioid receptors at higher doses. In addition to analgesia, the widely diverse effects of morphine sulfate include drowsiness, changes in mood, respiratory depression, decreased gastrointestinal motility, nausea, vomiting, and alterations of the endocrine and autonomic nervous system.
Effects on the Central Nervous System (CNS)
The principle therapeutic action of morphine is analgesia. Other therapeutic effects of morphine include anxiolysis, euphoria and feelings of relaxation. Although the precise mechanism of the analgesic action is unknown, specific CNS opiate receptors and endogenous compounds with morphine-like activity have been identified throughout the brain and spinal cord and are likely to play a role in the expression and perception of analgesic effects. In common with other opioids, morphine causes respiratory depression, in part by a direct effect on the brainstem respiratory centers. Morphine and related opioids depress the cough reflex by direct effect on the cough center in the medulla. Morphine causes miosis, even in total darkness.Effects on the Gastrointestinal Tract and on Other Smooth Muscle
Gastric, biliary and pancreatic secretions are decreased by morphine. Morphine causes a reduction in motility and is associated with an increase in tone in the antrum of the stomach duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone is increased to the point of spasm. The end result may be constipation. Morphine can cause a marked increase in biliary tract pressure as a result of spasm of the sphincter of Oddi. Morphine may also cause spasm of the sphincter of the urinary bladder.Effects of the Cardiovascular System
In therapeutic doses, morphine does not usually exert major effects on the cardiovascular system. Morphine produces peripheral vasodilation which may result in orthostatic hypotension and fainting. Release of histamine can occur, which may play a role in opioid-induced hypotension. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes, and sweating.Endocrine System
Opioid agonists have been shown to have a variety of effects on the secretion of hormones. Opioids inhibit the secretion of ACTH, cortisol, and luteinizing hormones (LH) in humans. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon in humans and other species, rats and dogs. Thyroid stimulating hormone (TSH) has been shown to be both inhibited and stimulated by opioids.Immune System
Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown.12.2 Pharmacodynamics
Morphine concentrations are not predictive of analgesic response, especially in patients previously treated with opioids. The minimum effective concentration varies widely and is influenced by a variety of factors, including the extent of previous opioid use, age and general medical condition. Effective doses in tolerant patients may be significantly higher than in opioid-naïve patients.
12.3 Pharmacokinetics
Morphine has an apparent volume of distribution ranging from 1.0 to 4.7 L/kg after parenteral administration. Protein binding is low, about 36%, and muscle tissue binding is reported as 54%. A blood-brain barrier exists, and when morphine is introduced outside of the CNS, plasma concentrations of morphine remain higher than the corresponding CSF morphine levels.
Morphine has a total plasma clearance which ranges from 0.9 to 1.2 L/kg/h in postoperative patients, but shows considerable interindividual variation. The major pathway of clearance is hepatic glucuronidation to morphine-3-glucuronide, which is pharmacologically inactive. The major excretion path of the conjugate is through the kidneys, with about 10% in the feces. Morphine is also eliminated by the kidneys, 2 to 12% being excreted unchanged in the urine. Terminal half-life is commonly reported to vary from 1.5 to 4.5 hours, although the longer half-lives were obtained when morphine levels were monitored over protracted periods with very sensitive radioimmunoassay methods. The accepted elimination half-life in normal subjects is 1.5 to 2 hours.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Studies in animals to evaluate the carcinogenic potential of morphine have not been conducted.Mutagenesis
No formal studies to assess the mutagenic potential of morphine have been conducted. In the published literature, morphine was found to be mutagenic in vitro increasing DNA fragmentation in human T-cells. Morphine was also reported to be mutagenic in the in vivo mouse micronucleus assay and positive for the induction of chromosomal aberrations in mouse spermatids and murine lymphocytes. Mechanistic studies suggest that the in vivo clastogenic effects reported with morphine in mice may be related to increases in glucocorticoid levels produced by morphine in these species. In contrast to the above positive findings, in vitro studies in the literature have also shown that morphine did not induce chromosomal aberrations in human leukocytes or translocations or lethal mutations in Drosophila.Impairment of Fertility
No formal nonclinical studies to assess the potential of morphine to impair fertility have been conducted. Several nonclinical studies from the literature have demonstrated adverse effects on male fertility in the rat from exposure to morphine. One study in which male rats were administered morphine sulfate subcutaneously prior to mating (up to 30 mg/kg twice daily) and during mating (20 mg/kg twice daily) with untreated females, a number of adverse reproductive effects including reduction in total pregnancies, higher incidence of pseudopregnancies, and reduction in implantation sites were seen. Studies from the literature have also reported changes in hormonal levels (i.e. testosterone, luteinizing hormone, serum corticosterone) following treatment with morphine. These changes may be associated with the reported effects on fertility in the rat. -
16 HOW SUPPLIED/STORAGE AND HANDLING
Morphine sulfate injection, USP is supplied in single-dose 1 mL vials for intravenous administration:
4 mg/mL packaged in 25s (NDC: 72572-440-25)
Store at 20ºC to 25°C (68°F to 77°F) [See USP Controlled Room Temperature] until ready to use. PROTECT FROM LIGHT. DO NOT FREEZE. Contains no preservative or antioxidant. DISCARD ANY UNUSED PORTION. DO NOT HEAT-STERILIZE.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if color is darker than pale yellow, if it is discolored in any other way, or if it contains a precipitate.
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For Product Inquiry call 1-877-845-0689.
-
17 PATIENT COUNSELING INFORMATION
Patients receiving parenteral morphine should be given the following instructions by the physician:
- The most common adverse events that may occur while taking morphine include nausea, somnolence, lightheadedness, dizziness, sedation, vomiting, diaphoresis, and constipation.
- Morphine analgesics may produce orthostatic hypotension in ambulatory patients.
- There is potential for severe constipation; appropriate laxatives and/or stool softeners as well as other appropriate treatments should be initiated from the onset of opioid therapy.
- Analgesic doses of morphine cloud judgment and impair the mental and/or physical abilities which are required for the performance of tasks such as driving a vehicle or operating machinery.
- Morphine will add to the effect of alcohol and other CNS depressants, including sedatives, hypnotics, tranquilizers, phenothiazines and antihistamines.
Mfd for: Civica, Inc.
Lehi, Utah 84043Mfd by: Hikma Pharmaceuticals USA Inc.
Cherry Hill, New Jersey 08003Revised August 2019
462-802-00
- PRINCIPAL DISPLAY PANELS
- SERIALIZATION IMAGE
-
INGREDIENTS AND APPEARANCE
MORPHINE SULFATE
morphine sulfate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72572-440 Route of Administration INTRAVENOUS DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MORPHINE SULFATE (UNII: X3P646A2J0) (MORPHINE - UNII:76I7G6D29C) MORPHINE SULFATE 4 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 0.2 mg in 1 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.4 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72572-440-25 25 in 1 PACKAGE 11/11/2019 1 NDC: 72572-440-01 1 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205758 11/11/2019 Labeler - Civica, Inc. (081373942) Registrant - Hikma Pharmaceuticals USA Inc. (946499746)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.


