VIONEXUS- chloroxylenol liquid
VioNexus by
Drug Labeling and Warnings
VioNexus by is a Otc medication manufactured, distributed, or labeled by Metrex Research, LLC, Metrex Research. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Drug Facts
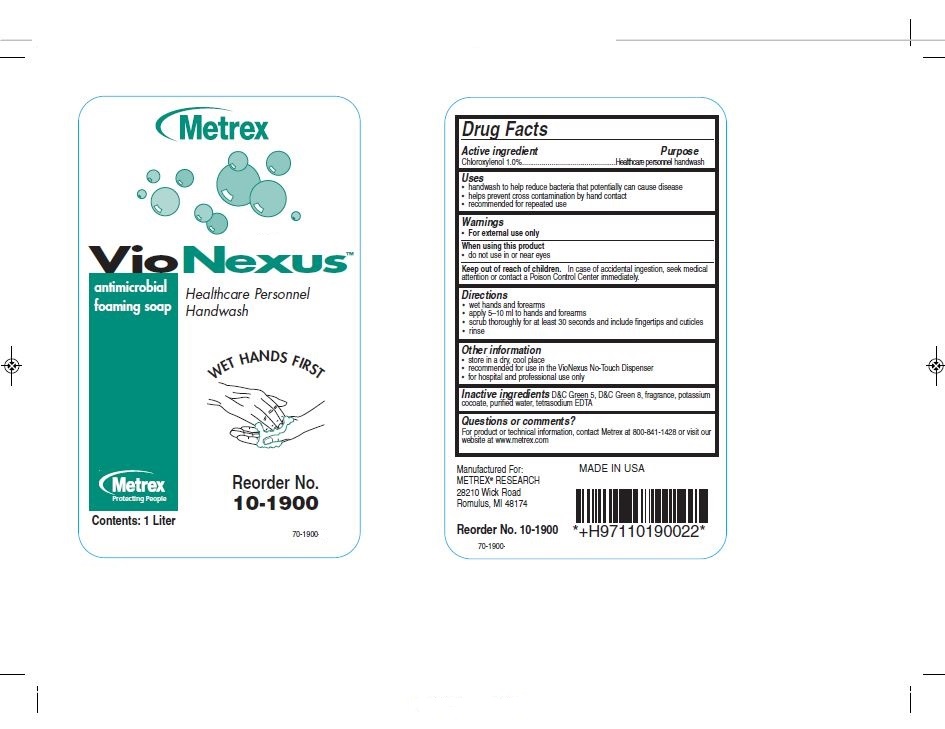 Active Ingredients Purpose
Active Ingredients Purpose
Chloroxylenol 1.0%................Healthcare personnel handwash
Uses
- handwash to help reduce bacteria that potentially can cause disease
- helps prevent cross contamination by hand contact
- recommended for repeated use
Keep out of reach of children
In case of accidental ingestion, seek medical attention or contact a poison control center immediately.
Directions
- wet hands and forearms
- apply 5-10 ml to hands and forearms
- scrub thoroughly for at least 30 seconds and include fingertips and cuticles
- rinse
- Principal Display Panel
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VIONEXUS
chloroxylenol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 55443-0600 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) EDETATE SODIUM (UNII: MP1J8420LU) D&C GREEN NO. 8 (UNII: I2W85YOX9L) D&C GREEN NO. 5 (UNII: 8J6RDU8L9X) POTASSIUM COCOATE (UNII: F8U72V8ZXP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55443-0600-3 3 in 1 CASE 10/01/2019 1 NDC: 55443-0600-2 2 in 1 BOX 1 NDC: 55443-0600-1 1000 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 10/01/2019 Labeler - Metrex Research (102567567) Registrant - Metrex Research (102567567)
Trademark Results [VioNexus]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VIONEXUS 85697923 4316679 Live/Registered |
Metrex Research, LLC 2012-08-08 |
 VIONEXUS 85494011 not registered Dead/Abandoned |
Metrex Research, LLC 2011-12-13 |
 VIONEXUS 76127921 not registered Dead/Abandoned |
Metrex Research Corporation 2000-09-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.


