ZYMFENTRA- infliximab-dyyb kit ZYMFENTRA- infliximab-dyyb injection
ZYMFENTRA by
Drug Labeling and Warnings
ZYMFENTRA by is a Prescription medication manufactured, distributed, or labeled by CELLTRION USA Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ZYMFENTRA safely and effectively. See full prescribing information for ZYMFENTRA.
ZYMFENTRA (infliximab-dyyb) injection, for subcutaneous use
Initial U.S. Approval: 2016WARNING: SERIOUS INFECTIONS and MALIGNANCY See full prescribing information for complete boxed warning Increased risk of serious infections leading to hospitalization or death, including tuberculosis (TB), bacterial sepsis, invasive fungal infections (such as hitoplasmosis) and infections due to ohter opportunistic pathogens. (5.1) Discontinue ZYMFENTRA if a patient develops a serious infection or sepsis. (5.1) Perform test for latent TB; if positive, start treatment for TB prior to starting ZYMFENTRA. Monitor all patients for active TB during treatment, even if initial latent TB test is negative. (5.1) Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with tumor necrosis factor (TNF) blockers, including infliximab products. (5.2) Postmarketing cases of fatal hepatosplenic T-cell lymphoma (HSTCL) have been reported in patients treated with TNF blockers including infliximab products. Almost all had received azathioprine or 6-mercaptopurine concomitantly with a TNF blocker at or prior to diagnosis. The majority of cases were reported in patients with Crohn's disease or ulcerative colitis, most of whom were adolescent or young adult males. (5.2 )
INDICATIONS AND USAGE
ZYMFENTRA is a tumor necrosis factor (TNF) blocker indicated in adults for maintenance treatment of:
- moderately to severely active ulcerative colitis following treatment with an infliximab product administered intravenously. (1)
- moderately to severely active Crohn’s disease following with an infliximab products administered intraneously. (1)
DOSAGE AND ADMINISTRATION
Important Dosage Information. (2.1).
- ZYMFENTRA is indicated as maintenance treatment only, starting at Week 10 and thereafter.
▫ All pateitns must complete an intravenous induction regiemen with an infliximab product before starting ZYMFENTRA. - ZYMFENTRA is for subcutaneous use only.
Recommeneded Maintenance Dosage in Ulcerative Colitis and Crohn's Direase (2.2)
- Week 10 and thereafter: Inject 120 mg subcutaneously once every two weeks.
- To swift patients who are responding to maintenance therapy with an infliximab product administered intravenously, administer the first subcutaneous dose of ZYMFENTRA in place of the next scheduled intravenous infusion and every two weeks thereafter.
- See the full prescribing information on how to administer subcutaneously. (2.3)
DOSAGE FORMS AND STRENGTHS
Injection (3):
- 120 mg/mL in a single-dose prefilled syringe.
- 120 mg/mL in a single-dose prefilled syringe with needle guard.
- 120 mg/mL in a single-dose prefilled pen.
CONTRAINDICATIONS
- History of severe hypersentitivity reaction to infliximab-dyyb, other infliximab products, any of the inactive ingredients in ZYMFENTRA or to any murine proteins. (4)
WARNINGS AND PRECAUTIONS
- Serious injections, including invasive fungal infections: Avoid use of ZYMFENTRA in patients with an active infection. If infection develops during treatment, conduct a prompt and complete diagnostic workup appropriate for an immunocompromised patient and initiate antimicrobial therapy. If systemic illness develops in patients who reside or travel to regions where mycoses are endemic, consider empiric antifungal therapy. (5.1)
- Malignancies: The incidence of malignancies, including lymphoma, was greater in TNF blocker-treated patients than in controls. Consider the higher risk of hepatosplenic T-cell lymphoma (HSTCL) with combination therapy versus increased tisk of immunogenicity and hypersensitivity reactions with monotherapy. (5.2)
- Hepatitis B virus (HBV) reactivation: Test for HBV infection before starting ZYMFENTRA. Monitor HBV carriers during and several months after therapy for active HBV infection. If reactivation occurs, stop ZYMFENTRA and begin anti-viral therapy. (5.3)
- Hepatotoxicity: Severe hepatic reactions, some fatal or necessitating liver transplantation with infliximab products. Monitor hepatic enzymes and liver function tests every 3 to 4 months during treatment; investigate liver enzyme elevations and interrupt treatment if drug-induced liver injury is suspected. Instruct patients to seek immediate medical attention if symptoms develop. (5.4)
- Congestive heart failure (CHF): New onset or worsening symptoms may occur. Avoid ZYMFENTRA in patients with CHF. Monitor for new or worsening symptoms if a decision is made to administer ZYMFENTRA. (5.5)
- Hematologic Reactions: Advise patients to seek immediate medical attention if signs and symtoms of cytopenia develop; consider stopping ZYMFENTRA if significant hematologic abnormalities devlop. (5.6)
- Hypersensitivity and Other Administration Reactions: Serious systemic hypersensitivity reactions including anaphylaxis; institute appropriate therapy and discontinue ZYMFENTRA. (4, 5.7)
- Neurologic Reactions: Exacerbation or new onset CNS demyelinating disorders may occurs; consider discontinuation of ZYMFENTRA. (5.8)
- Risk of infection with concurrent administration of other biological products: Concurrent use with other immunosuppressive biological products may increase the risk of injection (5.9)
- Risk of additive immunosuppressive effects from prior biologic products: Consider the half-life and mode of action of prior biological products. (5.10)
- Autoimmunity: Formation of autoantibodies and development of lupus-like syndrome may occur; discontinue ZYMFENTRA if symptoms develop. (5.11)
- Vaccinations and Use of Live Vaccines/Therapeutic Infectious Agents: Prior to initiating ZYMFENTRA bring patients up to date with all vaccinations. Live vaccines or therapeutic infectious agents should not be given with ZYMFENTRA. A 6-month waiting period following birth is recommended before the administration of live vaccines to infants exposed in utero to infliximab products. (5.12)
ADVERSE REACTIONS
Most common adverse reactions (>3%) are:
- Ulcerative Colitis: COVID-19, anemia, arthralgia, injection site reaction, increased alanine aminotransferase, and abdominal pain. (6.1)
- Crohn's Disease: COVID-19, headache, upper respiratory tract infection, injection site reaction, diarrhea, increased blood creatine phosphokinase, arthralgia, increased alanine aminotransferase, hypertension, urinary tract infection, neutropenia, dizziness, and leukopenia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact CELLTRION USA, Inc. at 1-800-560-9414 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
2.2 Recommeneded Dosage for Maintenance Treatment in Ulcerative Colitis Crohn's Disease
2.3 Subcutaenous Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
5.2 Malignancies
5.3 Hepatitis B Virus Reactivation
5.4 Hepatotoxicity
5.5 Congestive Heart Failure
5.6 Hematologic Reactions
5.7 Hypersensitivity and Other Administration Reactions
5.8 Neurologic Reactions
5.9 Risk of Infection with Concurrent Administration with Other Biological Products
5.10 Risk of Additive Immunosuppressive Effects from Prior Biological Products
5.11 Autoimmunity
5.12 Vaccinations and Use of Live Vaccines/Therapeutic Infectious Agents
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Other Biological Products Used to Treat UC and CD
7.2 Cytochrome P450 Substrates
7.3 Live Vaccines/Therapeutic Infectious Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatirc Use
11 DESCRIPTION
12 CLINICAL PHAMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Phamacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adult Ulcerative Colitis
14.2 Adult Crohn's Disease
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
SERIOUS INFECTIONS
Patients treated with TNF blockers, inculding ZYMFENTRA, are at increased risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions (5.1), Adverse Reactions (6.1)]. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
Discontinue ZYMFENTRA if a patient develops a serious infection or sepsis.
Reported infections include:
- Active tuberculosis, including reactivation of latent tuberculosis. Patients with tuberculosis have frequently presented with disseminated or extrapulmonary disease. Test patientsfor latent tuberculosis before ZYMFENTRA use and during therapy. Initiate treatment for latent infection prior to ZYMFENTRA use.
- Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Consider empiric anti-fungal therapy in patients at risk for invasive fungal infections who develop severe systemic illness.
- Bacterial, viral and other infections due to opportunistic pathogens, including Legionella and Listeria.
Carefully consider the risks and benefits of treatment with ZYMFENTRA prior to initiating therapy in patients with chronic or recurrent infection.
Closely monitor patients for the development of signs and symtoms of infection during and after treatment with ZYMFENTRA, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
MALIGNANCY
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including infliximab products [see Warnings and Precautions (5.2)].
Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma, have been reported in patients treated with TNF blockers, including infliximab products. These cases have had a very aggressive disease course and have been fatal. Almost all patients had received treatment with azathioprine or 6-mercaptopurine concomitantly with a TNF blocker at or prior to diagnosis. The majority of reported cases have occurred in patients with Crohn’s disease or ulcerative colitis and most were in young adult males. [see Use in Specific Population (8.4)].
-
1 INDICATIONS AND USAGE
ZYMFENTRA is indicated in adults for maintenance treatment of:
- moderately to severely active ulcerative colitis following treatment with an infliximab product administered intravenously.
- moderately to severely active Crohn's disease following treatment with an infliximab product administered intravetnously.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
- ZYMFENTRA is indicated as maintenance treatment only, starting at Week 10 and thereafter.▫ All patients mush coplete an intravenous induction regimen with an infliximab product before starting ZYMFENTRA. For induction dosing information, see the corresponding full prescribing information for the chosen infliximab product.
- ZYMFENTRA is for subtutaneous use only.
2.2 Recommeneded Dosage for Maintenance Treatment in Ulcerative Colitis Crohn's Disease
- Maintenance dosage starting at Week 10 and thereafter: 120 mg subcutaneously once every two weeks. To switch patients who are responding to maintenance therapy with an infliximab product administered intravenously, administer the first subcutaneous dose of ZYMFNETRA in place of the next scheuled intravenous infusion and every two weeks thereafter.
2.3 Subcutaenous Administration Instructions
- ZYMFENTRA is intended for use under the guidance and supervision of a healthcare professional.
- If a healthcare professional determines that it is appropriate, patients may self-injection ZYMFENTRA or caregivers may injection ZYMFENTRA using either the ZYMFENTRA prefilled syringe, ZYMFENTRA prefilled syringe with needle guard, or ZYMFENTRA prefilled pen after proper training in subcutaenous injection technique.
- Parental drug products should be inspected visually for particulate matter and discoloration prior to administration, whether solution and container permit. ZYMFENTRA should be a clear, colorless to pale brwon solution. Do not use if particulates or discoloration is presnet.
- Inject into the front of the tights, the abdomen excepts for the 2 inches around the navel, or the outer area of the upper arms (caregiver only).
- Rotate the injectoin site each time an inejction is given. Allow at least 1.2 inches between the new injection site and the previous inejction site. Never inject into areas where the skin is red, bruised, tender, or indurated.
- Do not use the syringe or pen of it has been dropped or is visibly damaged. A damaged syringe may not function proprely.
- Do not resue or shake the syringe or pen at any time.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
ZYMFENTRA is contraindicated in patients with a history of a severe hypersensitivity reaction to infliximab-dyyb, other infliximab products, any of the inactive ingredients in ZYMFENTRA, or any murine proteins. Reactions have included anaphylaxis [see Warnings and Precautions (5.7)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
Patients treated with ZYMFENTRA are at increased risk for developing serious infections involving various organ systems and sites that may lead to hospitalization or death.
Opportunistic infections due to bacterial, mycobacterial, invasive fungal, viral, or parasitic organisms including aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, cryptococcosis, histoplasmosis, legionellosis, listeriosis, pneumocystosis, salmonellosis and tuberculosis have been reported with TNF blockers. Patients have frequently presented with disseminated rather than localized disease.
Treatment with ZYMFENTRA should not be initiated in patients with an active infection, including clinically important localized infections.Patients greater than 65 years of age, patients with comorbid-conditions and/or patients taking concomitant immunosuppressants such as corticosteroids or methotrexate may be at greater risk of infection. The risks and benefits of treatment should be considered prior to initiating therapy in patients:
- with chronic or recurrent infection;
- who have been exposed to tuberculosis;
- with a history of an opportunistic infection;
- who have resided or traveled in areas of endemic tuberculosis or endemic mycoses, such as histoplasmosis, coccidioidomycosis, or blastomycosis; or
- with underlying conditions that may predispose them to infection.
Tuberculosis
Cases of reactivation of tuberculosis or new tuberculosis infections have been observed in patients receiving TNF-blockers, including patients who have previously received treatment for latent or active tuberculosis. Cases of active tuberculosis have also occurred in patients being treated with infliximab products during treatment for latent tuberculosis. Evaluate patients for tuberculosis risk factors and test for latent infection prior to initiating ZYMFENTRA and periodically during therapy.
Treatment of latent tuberculosis infection prior to therapy with TNF blockers has been shown to reduce the risk of tuberculosis reactivation during therapy. Induration of 5 mm or greater with tuberculin skin testing should be considered a positive test result when assessing if treatment for latent tuberculosis is needed prior to initiating ZYMFENTRA even for patients previously vaccinated with Bacille Calmette-Guérin (BCG).
Consider anti-tuberculosis therapy prior to initiation of ZYMFENTRA in patients with a past history of latent or active tuberculosis in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent tuberculosis but having risk factors for tuberculosis infection. Consultation with a physician with expertise in the treatment of tuberculosis is recommended to aid in the decision whether initiating anti-tuberculosis therapy is appropriate for an individual patient.
Tuberculosis should be strongly considered in patients who develop a new infection during treatment with ZYMFENTRA especially in patients who have previously or recently traveled to countries with a high prevalence of tuberculosis, or who have had close contact with a person with active tuberculosis.
Monitoring
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with ZYMFENTRA including the development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy. Tests for latent tuberculosis infection may also be falsely negative while on therapy with ZYMFENTRA.
Discontinue ZYMFENTRA if a patient develops a serious infection or sepsis. A patient who develops a new infection during treatment with ZYMFENTRA should undergo prompt and complete diagnostic workup appropriate for an immunocompromised patient; and appropriate antimicrobial therapy should be initiated, and the patient should be closely monitored.
Invasive Fungal Infections
For patients who reside or travel in regions where mycoses are endemic, invasive fungal infection should be suspected if they develop a serious systemic illness. Appropriate empiric antifungal therapy should be considered while a diagnostic workup is being performed. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. When feasible, the decision to administer empiric antifungal therapy in these patients should be made in consultation with a physician with expertise in the diagnosis and treatment of invasive fungal infections and should take into account both the risk for severe fungal infection and the risks of antifungal therapy.
5.2 Malignancies
Malignancies, some fatal, have been reported among children, adolescents and young adults who received treatment with TNF blockers (initiation of therapy ≤18 years of age), including infliximab products. AApproximately half of these cases were lymphomas, including Hodgkin’s and non-Hodgkin’s lymphoma. The other cases represented a variety of malignancies, including rare malignancies that are usually associated with immunosuppression and malignancies that are not usually observed in children and adolescents. The malignancies occurred after a median of 30 months (range 1 to 84 months) after the first dose of TNF blocker therapy. Most of the patients were receiving concomitant immunosuppressants. These cases were reported postmarketing and are derived from a variety of sources, including registries and spontaneous postmarketing reports.
Lymphomas
In the controlled portions of clinical trials of TNF blockers, more cases of lymphoma have been observed among patients receiving a TNF blocker compared with control patients. Cases of acute and chronic leukemia have been reported with postmarketing TNF blocker use, including infliximab products.
Hepatosplenic T-cell Lymphoma (HSTCL)
Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma, have been reported in patients treated with TNF blockers, including infliximab products. TThese cases have had a very aggressive disease course and have been fatal. Almost all patients had received treatment with the immunosuppressants azathioprine or 6-mercaptopurine concomitantly with a TNF blocker at or prior to diagnosis. The majority of reported cases have occurred in patients with Crohn’s disease or ulcerative colitis and most were in adolescent and young adult males. It is uncertain whether the occurrence of HSTCL is related to TNF blockers or TNF blockers in combination with these other immunosuppressants. When treating patients, consideration of whether to use ZYMFENTRA alone or in combination with other immunosuppressants such as azathioprine or 6-mercaptopurine should take into account a possibility that there is a higher risk of HSTCL with combination therapy versus an observed increased risk of immunogenicity and hypersensitivity reactions with TNF blocker monotherapy from the clinical trial data [see Warnings and Precautions (5.7)]
Skin Cancer
Melanoma and Merkel cell carcinoma have been reported in patients treated with TNF blocker therapy, including infliximab products. Periodic skin examination is recommended for all patients during treatment with ZYMFENTRA, particularly those with risk factors for skin cancer.
Cervical Cancer
Cases of invasive cervical cancer have been reported postmarketing in women who received infliximab products for other conditions. A causal relationship between infliximab products and cervical cancer cannot be excluded. Routine cervical cancer screening is recommended during treatment with ZYMFENTRA.
Other Malignancies
In the controlled pportions of clinical trials of some TNF blockers, including infliximab products, more malignancies (excluding lymphoma and nonmelanoma skin cancer) have been observed in patients receiving those TNF blockers compared with control patients. The most common malignancies were breast, colorectal, and melanoma in these controlled trials of TNF blockers.
In controlled trials of TNF blockers in adult patients at higher risk for malignancies (i.e., patients with COPD with a significant smoking history and cyclophosphamide-treated patients with Wegener’s granulomatosis), a greater proportion of malignancies occurred in the TNF blocker group compared to the control group. Patients had a history of heavy smoking. Avoid ZYMFENTRA in patients with moderate to severe COPD.
The potential role of TNF blockers in the development of malignancies is not known. Avoid ZYMFENTRA treatment in patients with a history of malignancy or in continuing treatment in patients who develop malignancy while receiving ZYMFENTRA.
5.3 Hepatitis B Virus Reactivation
Use of TNF blockers has been associated with reactivation of hepatitis B virus (HBV) in patients who are chronic carriers of this virus. In some instances, HBV reactivation occurring in conjunction with TNF blocker therapy has been fatal. The majority of these reports have occurred in patients concomitantly receiving other medications that suppress the immune system, which may also contribute to HBV reactivation.
Test patients for HBV infection before initiating TNF blocker therapy, including ZYMFENTRA. For patients who test positive for hepatitis B surface antigen, consultation with a physician with expertise in the treatment of hepatitis B is recommended. Adequate data are not available on the safety or efficacy of treating patients who are carriers of HBV with antiviral therapy in conjunction with TNF blocker therapy to prevent HBV reactivation. Patients who are carriers of HBV and require treatment with TNF blockers, including ZYMFENTRA, should be closely monitored for clinical and laboratory signs of active HBV infection throughout therapy and for several months following termination of therapy.
In patients who develop HBV reactivation, discontinue ZYMFENTRA and initiate antiviral therapy with appropriate supportive treatment. The safety of resuming TNF blocker therapy after HBV reactivation is controlled is not known. Therefore, prescribers should exercise caution when considering resumption of ZYMFENTRA in this situation and monitor patients closely.
5.4 Hepatotoxicity
Hepatobiliary disorders, including acute liver failure, jaundice abnormal hepatic function, hepatic steatosis, hepatitis, hepatotoxicity, hyperbilirubinemia and non-alcoholic fatty liver, have been reported in postmarketing data in patients receiving infliximab products. Autoimmune hepatitis has been diagnosed in some of these cases. Severe hepatic reactions occurred between two weeks to more than one year after initiation of infliximab products administered intravenously; elevations in hepatic aminotransferase levels were not noted prior to discovery of the liver injury in many of these cases. Some of these cases were fatal or necessitated liver transplantation.
In clinical trials, three subjects treated with ZYMFENTRA had drug induced liver injury based on hepatic transaminase elevations, including one subject with accompanying bilirubin elevation [see Adverse Reactions (6.2)].
Monitor hepatic enzymes and liver function tests every 3 to 4 months during treatment with ZYMFENTRA. Prompt investigation of the cause of liver enzyme elevation should be undertaken to identify potential cases of drug-induced liver injury. Interrupt treatment if drug-induced liver injury is suspected, until this diagnosis is excluded. Instruct patients to seek immediate medical attention if they experience symptoms suggestive of hepatic dysfuction.
5.5 Congestive Heart Failure
Cases of worsening congestive heart failure (CHF) and new onset CHF, with and without identifiable precipitating factors (e.g., pre-existing cardiovascular disease), have been reported with TNF blockers, including infliximab products. Some of these patients have been under 50 years of age, and some cases had a fatal outcome. In several exploratory trials of other TNF blockers in the treatment of CHF, there were greater proportions of TNF-blocker-treated patients who had CHF exacerbations requiring hospitalization or increased mortality. ZYMFENTRA has not been studied in patients with a history of CHF. Avoid ZYMFENTRA in patients with CHF.
If a decision is made to administer ZYMFENTRA to patients with CHF, closely monitor patients during therapy for new or worsening symptoms of heart failure and discontinue ZYMFENTRA if symptoms appear.
5.6 Hematologic Reactions
Report of pancytopenia including aplastic anemia have been reported with TNF blocking agents. Cases of leukopenia, neutropenia, thrombocytopenia, and pancytopenia, some with a fatal outcome, have been reported in patients receiving infliximab products. Clinically significant events of neutropenia were noted in the clinical trials of ZYMFENTRA. The causal relationship to infliximab product therapy remains unclear. Although no high-risk group(s) has been identified, avoid ZYMFENTRA in patients who have ongoing or a history of significant hematologic abnormalities. Advise all patients to seek immediate medical attention if they develop signs and symptoms suggestive of blood dyscrasias or infection (e.g., persistent fever) during treatment with ZYMFENTRA. Consider discontinuation of ZYMFENTRA therapy in patients who develop significant hematologic abnormalities.
5.7 Hypersensitivity and Other Administration Reactions
In clinical trials of ZYMFENTRA, symptoms compatible with hypersensitivity reactions have been reported including bronchospasm, dyspnea, rash, and edema. In post-marketing experience, serious systemic hypersensitivity reactions (including anaphylaxis, hypotension, and serum sickness) have been reported following administration of infliximab products. If an anaphylactic or other clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue ZYMFENTRA. There are no data on the risks of using ZYMFENTRA in patients who have experienced a severe hypersensitivity reaction towards another TNF blocker; in these patients, caution is needed.
5.8 Neurologic Reactions
Agents that inhibit TNF have been associated with central nervous system (CNS) manifestation of systemic vasculitis, seizure and new onset or exacerbation of clinical symptoms and/or radiographic evidence of CNS demyelinating disorders, including multiple sclerosis and optic neuritis, and peripheral demyelinating disorders, including Guillain-Barré syndrome. Avoid the use of ZYMFENTRA in patients with these neurologic disorders and consider discontinuation of ZYMFENTRA if these disorders develop.
5.9 Risk of Infection with Concurrent Administration with Other Biological Products
Serious infections and neutropenia have been reported with concurrent use of TNF-blockers and other immunosuppressive biological products (e.g., anakinra and abatacept). The concurrent use of ZYMFENTRA with other immunosuppressive biological products used to treat ulcerative colitis and Crohn’s disease may increase the risk of infection and is not recommended [see Drug Interactions (7.1)].
5.10 Risk of Additive Immunosuppressive Effects from Prior Biological Products
Consider the half-life and mode of action of prior biological products to avoid unintended additive immunosuppressive effects when initiating ZYMFENTRA [see Drug Interactions (7.1)].
5.11 Autoimmunity
Treatment with TNF blockers, iincluding ZYMFENTRA may result in the formation of autoantibodies and in the development of a lupus-like syndrome. If a patient develops symptoms suggestive of a lupus-like syndrome following treatment with ZYMFENTRA, discontinue treatment.
5.12 Vaccinations and Use of Live Vaccines/Therapeutic Infectious Agents
Vaccinations
Prior to initiating ZYMFENTRA in adult patients, update vaccinations in accordance with current vaccination guidelines.
Live Vaccines and Theraeputic Infectious Agents
In patients receiving TNF blockers, limited data are available on the response to vaccination with live vaccines or on the secondary transmission of infection by live vaccines. Use of live vaccines can result in clinical infections, including disseminated infections. The concurrent administration of live vaccines with ZYMFENTRA is not recommended.
Fatal outcome due to disseminated BCG infection has been reported in an infant who received a BCG vaccine after in utero exposure to infliximab products. Infliximab is known to cross the placenta and has been detected in the serum of infants up to 6 months following birth. A 6-month waiting period following birth is recommended before the administration of any live vaccine to infants exposed in utero to infliximab products.
Other uses of therapeutic infectious agents such as live attenuated bacteria (e.g., BCG bladder instillation for the treatment of cancer) could result in clinical infections, including disseminated infections. It is recommended that therapeutic infectious agents not be given concurrently with ZYMFENTRA.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Serious infections [seeWarnings and Precautions (5.1)]
- Malignancies [see Warnings and Precautions (5.2)]
- Hepatitis B virus reactivation [seeWarnings and Precautions (5.3)]
- Hepatotoxicity [seeWarning and Precautions (5.4)]
- Congestive heart failure [seeWarnings and Precautions (5.5)]
- Hematologic reactions [seeWarnings and Precautions (5.6)]
- Hypersensitivity and other administration reactions [seeWarnings and Precautions (5.7)]
- Neruologic reactions [seeWarnings and Precautions (5.8)]
- Autoimmunity [seeWarnings and Precautions (5.11)]
6.1 Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The data described below reflect exposure to ZYMFENTRA in 518 adult subjects in two 54-weekrandomized, double-blind, placebo-controlled trials in subjects with moderately to severely active ulcerative colitis (UC) or Crohn’s disease (CD) (UC Trial I an CD Trial I). Subject who achieved clinical response following three induction doses of infliximab-dyyb administered as an intravenous infusion at Week 0, 2 and 6 were randomized 2:1 to ZYMFENTRA 120 mg or placebo as a subcutaneous injection every two weeks at Week 10 [see Clinical Studies (14.1 and 14.2)].
Ulcerative Colitis
The most common adverse reactions reported in ≥3% of subjects and at a higher rate than Placebo in UC Trial I are shown in Table 1.
Table 1 Adverse Reactionsa in the Maintenance Phase of a Randomized, Double-Blind54-Week Study of Subjects with UC (UC Tral I)
ZYMFENTRA
120 mg Subcutaneous
Injectionb
N=296
(%)
Placebo
N=140
(%)
COVID-19
10 6
Anemiad
5
4
Arthralgia
4
1
Injection site reaction# 3 2 Increased alanine aminotransferase
3
1
Abominal painf
3
1
areported in at least 3% of ZYMFENTRA-treated subjects and at a higher than placebo
bZYMFENTRA 120 mg as a subcutaneous injection every two weeks starting Week 10 following 3 intravenous induction doses of inflixiab-dyyb
cIncludes: COVID-19 and COVID-19 pneumonia
dIncludes: anemia and iron deficiency anemia
#Some subjects had multiple occurences of injection site reactions. In this table, injection site reactions are counted only once per subject. Symptoms in individual subjects included one or more of injection site bruising, edema, erythema, induration, pain, pruritus and swelling.
fIncludes: abdominal pain, upper abdominal pain, lower abdominal pain, and abdominal discomfort
Crohn's Disease
The most common adverse reactions reported in ≥3% of subjects and at a higher rate than placebo in CD Trial I are shown in Table 2.
Table 2 Adverse Reactionsa in the Maintenance Phase of a Randomized, Double-Blind
54-Week Study of Subjects with CD (CD Trial I)ZYMFENTRA
120 mg Subcutaneous
Injectionb
N=222
(%)
Placebo
N=101
(%)
COVID-19
10 5
Headache
8 4
Upper respiratory track infectionsdc
7 3
Injection site reactiond
5 1
Diarrhea
5
1
Increased blood creatine phosphokinase
4
2
Arthralgia
4
3
Increased alaine aninotransferase
4
1
Hypertensionse 3 2 Urinary track infectionf 3 2 Neutropenia 3 0 Dizziness 3 0 Leukopenia
3
0
a reported in at least 3% of ZYMFENTRA-treated subjects and at a higher rate than placebo
b ZYMFENTRA 120 mg administered subcutaneously starting at Week 10 and every 2 weeks thereafter for up to Week 54.
c Includes: upper respiratory tract infection, acute sinusitis, chronic sinusitis, influenza like illness, nasopharyngitis, pharyngitis, pharyngitis streptococcal, rhinitis, rhinorrhea, rhinovirus infection, sinusitis, tonsillitis
d Some subjects had multiple occurrences of injection site reactions. In this table, injection site reactions are counted only once per subject. Symptoms in individual subjects included one or more of injection site bruising, edema, erythema, induration, pain, pruritus, rash, swelling, warmth.
e Includes: hypertension and essential hypertension
f Includes: urinary tract infection, pyelonephritis
Adverse Evetns of Special Interest
Infections
In UC Trial I, at leaset one serious infection was reported in 3% of ZYMFENTRA-treated subjects compared to 1% in the plaebo group. Serious infections in the ZYMFENTRA-treated subjects were COVID-19, cystitis, pneumonia, salpingitis, and urinary tract injection [seeWarnings and Precautions (5.1)].
In CD Trial I, infections were observed in 30% of ZYMFENTRA-treated subjects compare to 187% of placebo-treated subjects. At least one serious infection was reported in 3% of ZYMFENTRA-treated subjects compared to 1% in the placebo group. Serious infections in the ZYMFENTRA-treated subjects were abscess, appendicitis, bacterial arthritis, bartholinitis, bronchiolitis, and urinary tract infection [seeWarnings and Precautions (5.1)].
Malignancies
In UC Trial I, malignancy (prostate cancer) was reported in a ZYMFENTRA-treated subject. No malignancies were reported among placebo-treated subjects [seeWarnings and Precautions (5.2)].
In another clinical trial in patients with CD, one malignancy (non-small cell lung cancer) was reported in a ZYMFENTRA-treated subject [see Warnings and Precautions (5.2)].
Hepatotoxicity
Three cases of drug induced liver injury were noted in ZYMFENTRA-treated subjects that led to study drug discontinuation [see Warnings and Precautions (5.4)].
- In two subjects in UC Trial I, ALT and AST levels started rising 7 to 12 months after starting ZYMFENTRA, reaching peak values of 4 to 11x ULN for ALT, and 2 to 7x ULN for AST. Inboth subjects, total bilirubin levels remains below 2x ULN.
- In one subject in CD Trial I, ALT and AST started rising within a month after starting ZYMFENTRA, reaching peack values of 18x ULN for ALT and 14.9x ULN for AST. At approximately 5 months, total bilirubin level also increased to a peak value of 2.5x ULN.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of outside of the U.S with a non-U.S approved infliximab product administered subcutaneously. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Infections and infestations: cellulitis, disseminated tuberculosis, lower respiratory tract infection, pneumonia, sepsis
- Neoplasms benign, malignant and unspecified: breast cancer, gastric cancer, lung cancer
- Nervous system disorders: multiple sclerosis
- General disorders and administration site conditions: fatigue, malaise
The following additional adverse ractions have been identified during post-approval use of infliximab products administered intravenously.
- Neutropenia, agranuloxytosis (including infants exposed in utero to infliximab products), idiopathis thrombocytopenic purpura, thrombotic thrombocytopenic purpura.
- Interstitial lung disease (including pulmonary fibrosis/interstitial pneumonitis and rapidly progessive disease).
- Pericardial effusion, systemic and cutaneous vasculitis.
- Erythema multiforme, Stevens-Johnson Syndrome, toxic epidermal necrolysis, linear IgA bullous dermatosis (LABD), acute generalized exanthematous pustulosis (AGEP), new onset and worsening psoriasis (all subtypes including pustular, primarily palmoplantar), lichenoid react.
- Preipheral demyelinating disorders (such as Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, and multifocal motor neuropathy) transverse myelitis, and neuropathies (additional neurologic reactions have also been observed).
- Acute liver failure, jaundice, hepatitis, and cholestasis.
- Serious infections and vaccine breakthrough infection including bovine tuberculosis (disseminated BCG infection) following vaccination in an infant exposed in utero to infliximab products.
- Malignancies, including leukemia, melanoma, Merkel cell carcinoma, and cervical cancer.
- Anaphylactic reactions, including anaphylactic shock, laryngeal/pharyngeal edema and severe bronchospasm, and seizure.
- Transient visual loss has been reported in association with infliximab products during or within 2 hours of infusion. Cerebrovascular accidents, myocardial ischemia/infarction (some fatal), and arrhythmia occurring within 24 hours of initiation of infusion have also been reported.
-
7 DRUG INTERACTIONS
7.1 Other Biological Products Used to Treat UC and CD
The concurrent use of ZYMFENTRA with other immunosuppressive biological products used to treat UC and CD may increase the risk of infection and is not recommended [seeWarnings and Precautions (5.9)].
Consier the half-life and mode of action of prior biologial products to avoid unintended additive immunosuppressive effects when initiating ZYMFENTRA [seeWarnings and Precautions (5.10)].
7.2 Cytochrome P450 Substrates
The formation of CYP450 enzymes may be suppressed by increased levels of cytokines (e.g., TNFα, IL-1, IL-6, IL-10, IFN) during chronic inflammation. Therefore, ZYMFENTRA, an antagonist of TNFα, could normalize the formation of CYP450 enzymes potentially resulting in a decrease in exposure of CYP450 substrates.
Upon initiation or discontinuation of TNF blockers, including ZYMFENTRA, in patients being treated with CYP450 substrates requiring therapeutic drug monitoring, monitor therpeutic parameters (e.g., INR for warfarin) or drug concentration (e.g., cyclosporine or theophylline). Dosage adjustment may be needed to maintain drug concentrations or parameters within the therapeutic range. See prescribing information for specific drugs.
7.3 Live Vaccines/Therapeutic Infectious Agents
It is recommended that live vaccines not be given concurrently with ZYMFENTRA. It is also recommended that live vaccines not be given to infants after in utero exposure to infliximab products for 6 months following birth [see Warnings and Precautions (5.12)].
It is recommended that therapeutic infectious agents not be given concurrently with ZYMFENTRA [see Warnings and Precautions (5.12)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from reports of pregnancy in clinical trials with ZYMFENTRA are insufficient to identify a drug associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. There are risks to the mother and the fetus associated with inflammatory bowel disease
in pregnancy (see Clinical Considerations).Available observational data in pregnant women exposed to infliximab products administered intravenously showed no increased risk of major malformations among live births as compared to those exposed to non-biologics. Most TNF blockers, such as infliximab products, administered
intravenously are transferred across the placenta during the third trimester of pregnancy and may affect immune response in the in utero exposed infant. Infants exposed in utero should not be administered live vaccines for at least 6 months after birth (see Clinical Considerations). Because infliximab products do not cross-react with TNFα in species other than humans and chimpanzees, animal reproduction studies have not been conducted with ZYMFENTRA.The background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and
miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.Clinical Consideration
Disease-Associated Maternal and/or Embryo/Fetal Risk
Published data suggest that there is an increased risk of adverse pregnancy outcomes in women with inflammatory bowel disease associated with increased disease activity. Adverse pregnancy outcomes include preterm delivery (before 37 weeks of gestation), low birth weight (less than 2.5
kg) and small for gestational age at birth.Fetal/Neonatal Adverse Reactions
The risk of fetal/neonatal adverse reactions with in utero exposure to infliximab-dyyb administered subcutaneously is unknown. However, most TNF blockers, such as infliximab products, cross the placenta and have been detected in infant serum up to 6 months following birth. Consequently, infants exposed to infliximab products may be at increased risk of infection, including disseminated infection which can become fatal. At least a six-month waiting period following birth is recommended before the administration of live vaccines (e.g., BCG vaccine or other live vaccines, such as the rotavirus vaccine) to infants exposed to intravenous infliximab in utero [see Warnings and Precautions (5.12)].
Cases of agranulocytosis in infants exposed to intravenous infliximab in utero have also been reported. Therefore, ZYMFENTRA, administered during pregnancy may affect immune responses in the in utero-exposed newborn and infant [see Adverse Reactions 6.2].
8.2 Lactation
Risk Summary
There are no data on the presence of infliximab-dyyb or its metabolites in either human or animal milk, the effects on the breastfed infant, or the effects on milk production after subcutaneous administration. Published literature show that infliximab is present at low levels in human milk after intravenous administration. Systemic exposure in a breastfed infant is expected to be low because infliximab products are largely degraded in the gastrointestinal tract. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ZYMFNETRA and any potential adverse effects on the brestfed child from ZYMFENTRA or from the underlying maternal condition.
-
11 DESCRIPTION
Infliximab-dyyb, a tumor necrosis factor (TNF) blocker, is a chimeric IgG1κ monoclonal antibody (composed of human constant and murine variable regions). It has a molecular weight of approximately 145.9 kDa. Infliximab-dyyb is produced by a recombinant murine myeloma cell line, SP2/0.
ZYMFENTRA (inifliximab-dyyb) injection for subcutaneous use is a sterile, preservative-free, clear to opalescent, colorless to pale brown solution.
ZYMFENTRA is supplied in a single-dose prefilled syringe with 29 gauge fixed 1/2 inch needle, prefilled syringe with 29 gauge fixed 1/2 inch needle with needle guard, or prefilled pen with 27 gauge fixed 1/2 inch needle.
Each mL of solution contains 120 mg infliximab-dyyb, acetic acid (0.19 mg), polysorbate 80 (0.5 mg), sodium acetate (0.56 mg), sorbitol (45 mg) and Water for Injection, USP. The pH is 5.0.
-
12 CLINICAL PHAMACOLOGY
12.1 Mechanism of Action
Infliximab-dyyb neutralize the biological activity of TNFα by binding with high affinity to the soluble and transmembrane forms of TNFα and inhibit binding of TNFα with its receptors. Infliximab-dyyb has shown biological activities, such as TNFα neutralization activity and TNFα binding affinities, complement 1q (C1q) binding affinity and crystallizable fragment (Fc) receptor binding affinities in a wide variety of in vitro bioasays. The relationship of these biological response markers to the mechanism(s) by which infliximab-dyyb exerts its clinical effects is unknown.
12.3 Phamacokinetics
Following single and multiple subcutaneous dosing of infliximab-dyyb, exposures to infliximab-dyyb (i.e., AUC) are proportionally increased over the dose range from 120 mg to 240 mg (2 times the recommended dosage). After a single subcutaneous dose of infliximab-dyyb 120 mg in healthy subjects, the median (SD) Cmax and AUCinf were 10.0 (3.2) mcg/mL and 6945.6 (2830.2) mcg·h/mL, respectively. Following recommended subcutaneous maintenance dose of ZYMFENTRA (120 mg every 2 weeks) in adult subjects with UC and CD from Week 10 after intravenous induction treatment with infliximab-dyyb, steady-state was achieved by Week 22, and the mean (SD) trough serum concentrations of infliximab-dyyb at steady-state were 14.6 (7.8) mcg/mL and 14.6 (8.9) mcg/mL in subjects with UC and CD, respectively. Pharmacokinetics are comparable between healthy subjects, and subjects with UC or CD.
Absorption
In healthy subjects, the median time to reach the maximum serum concentration (Tmax) was 7 days after a single subcutaneous dose of infliximab-dyyb 120 mg. In healthy subjects, AUCinf following a single subcutaneous dosed of infliximab-dyyb 120 mg was approximately 23% relative to a single intravenous dose of infliximab-dyyb 5 mg/kg. Following infliximab-dyyb 120 mg subcuaneosly every 2 weeks, steady state AUC for 8-week internal was 25-35% higher compared to infliximab-dyyb 5 mg/kg administered intravenously every 8 weeks in subjects with UC and CD.
Distribution
Population pharmacokinetic analyses showed that the volume of distribution of infliximab-dyyb was 3.36L.
Elimination
In healthy subjects, the mean half-life was 332 hours after a single subcutaneous dose of infliximab-dyyb 120 mg.
Populatoin pharmacokinetic analyses showed that the clearance of infliximab-dyyb was 0.013 L/hr and the clearance was increased in the presence of anti-drug antibody.
The metabolic pathway of infliximab has not been characterized. As a IgG1κ monoclonal antibody, infliximab is expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG.
Specific Populations
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Difference in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of infliximab-dyyb or of other infliximab products.
In UC and CD clinical studies (UC Trial I and CD Trial I), approximately 64% subjects developed anti-drug antibodies to infliximab-dyyb following induction treatment with infliximab-dyyb intravenously and maintenance treatment with ZYMFENTRA by Week 54 [183/287 (64%) in subjects with UC, and 151/232 (65%) in subjects with CD] [see Clinical Studies (14)]. Among subjects with anti-drug antibodies, 92% had neutralizing antibodies [161/183 (88%) subjects with UC, and 147/151 (97%) subjects with CD]. Use of concomitant immunosuppressant agents (azathioprine, 6-mercaptopurine and methotrexate) appeared to reduce the frequency of ADA to infliximab-dyyb.
Anti-Drug Antibody Effects on Pharmacokinetics
Subjects who were positive for anti-drug antibodies showed lower infliximab-dyyb trough seum concentrations of infliximab-dyyb by approximately 30 to 40% compared to subjects who were negative for anti-drug antibodies. In some subjects with high titers of anti-drug antibodies and positive neutralizing antibodies, trough seum concentrations of infliximab-dyyb were below the lower limit of quantitation (<0.1 mcg/mL). There was no identified clinically significant effect of anti-drug antibodies on the safety and effectiveness of ZYMFENTRA in Study UC I and Study CD I.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Adult Ulcerative Colitis
The safety and efficacy of ZYMFENTRA were assessed in a randomized, double-blind, placebo-controlled clinical trial (UC Trial I; NCT04205643) in adult subjects with moderately to severely active UC (defined as a modified Mayo score [mMS] between 5 to 9 with an endoscopic subscore [ES] of 2 or 3). The mMS is a 3-component Mayo score (0-9), which consists of following subscores (0 to 3 for each subscore): stool frequency (SFS), rectal bleeding (RBS), and findings on centrally read endoscopy score (ES). An ES of 2 was defined by marked erythema, lack of vascular pattern, any friability, and/or erosions, and a score of 3 was defined by spontaneous bleeding and ulceration.
Subjects had demonstrated an inadequate response or intolerance to treatment with corticosteroids alone or in combination with 6-mercaptopurine or azathioprine. Subjects were permitted to use stable doses of oral aminosalicylates, oral corticosteroids (prednisone ≤20 mg/day or equivalent, budesonide ≤9 mg/day), UC-related antibiotics, and/or immunomodulatory agents (azathioprine, 6-mercaptopurine, or methotrexate). Corticosteroid tapering was permitted after Week 10.
All subjects received three intravenous induction doses of 5 mg/kg of infliximab-dyyb at Weeks 0, 2 and 6. In order to be randomized to treatment in UC Trial I, subjects had to be in clinical response at Week 10. Clinical response was defined as a decrease from baseline in the mMS of at least 2 points and at least 30%, with an accompanying decrease in the RBS of at least 1 point or an aabsolute RBS of 0 or 1 point.
A total of 438 subjects were randomized at Week 10 in a double-blind fashion (2:1) to ZYMFENTRA 120 mg as a subcutaneous injection or placebo every two weeks.
At the time of randomization into the double-blind phase (Week 10), 92% were receiving aminosalicylates, 41% were receiving oral corticosteroids, and 22% were receiving immunomodulators including AZA, 6-MP or MTX.
A total of 10% of randomized subjects had prior exposure to biological poucts or JAK inhibitors.
Subjects in the boule-blind phase had a mean age of 39 years (range 18 to 75 years); 44% were female; and 98% identified as White, and 2% as American Indian or Alaska Native.
The primary endpoint was the proportion of subjects in clinical remission at Week 54. Wecondary endpoints included the proportion of subjects achieving histologic-endoscopic mucosal improvement and corticosteroid-fee remission at Week 54 (see Table 3).
Table 3: Proportion of Subjects with Ulcerative Colitis Meeting Effficacy Endpoints at Week 54 in UC Trial I
Endpoint
ZYMFENTRA
Placebo
Treatment Differencea
and 95% CI
Clinical Remissionb at Week 54
Total population N=294
43%
N=144
21%
21%
(12, 29)
No prior biological product/JAK inhibitor exposure N=265
45%
N=131
21%
Prior biological product/JAK inhibitor exposure N=29
31%
N=13
15%
Histologic-Endoscopic Mucosal Improvementd at Week 54 Total population
N=294
36%
N=144
17%
18%
(9, 26)
No prior biological product/JAK inhibitor exposure N=265
36%
N=131
18%
Prior biological product/JAK inhibitor exposure N=29
31%
N=13
8%
Corticosteroid-Fee Remissione at Week 54 Total population N=120
37%
N=61
18%
17%f
(3, 29)
No prior biological product/JAK inhibitor
N=107
36%
N=56
18%
Prior biological product/JAK inhibitor exposure N=13
39%
N=5
20%
CI = confience interval, JAK = Janus kinase
a Treatement difference (adjusted for stratification factors of previous exposure to biological product and/or JAK inhibitor, use of treatment with oral corticosteoids Week 0, and clinical remission status at Week 10.)
b Clinical remissio is defined using the mMS as SFS of 0 or 1 point; RBS of 0 point; and ES of 0 or 1 point (excluding friability).
c p < 0.0001
d Histologic-endoscopic mucosal improvement is defined as an absolute ES of 0 or 1 point (excluding friability) from mMS and an absoluted Robarts Histopathology Index (RHI) score of ≤3 poitns with no lamina propria neutrophils, no neutrophils in epithelium, no erosion or ulceration.
e Corticosteroid-free remission is defined as being in clinical remission by mMS in addition to not requiring any treatment with corticosteroid for at least 8 weeks at Week 54, among the patients who used oral cotricosteroids at baseline.
f p < 0.05
The relationship between histologic-endoscopic mucosal improvement at Week 54 and diasease progression and longer-term outcomes after Week 54 was not evaluated in UC Trial I.
14.2 Adult Crohn's Disease
The safety and efficacy of ZYMFENTRA were assessed in a randomized, double-blind, placebo-controlled clinical studies (CD Trial I; NCT03945019) in adult subjects with moderately to severely active CD, defined as Crohn's Disease Activity Index (CDAI) score of 220 to 450 points, and a centrally-reveiwed Simplified Endoscopic Activity Score for Crohn’s Disease (SES-CD) of ≥6 points for ileal-colonic CD (or ≥4 points for isolated ileal disease).
Subjects had demonstrated an inadequate response or intolerance to treatment with corticosteroids and/or immunosuppressants. Subjects were permitted to use stable doses of oral amisosalicylates, oral corticosteroids (prednisone ≤20 mg/day or equivalent, budesonide ≤9 mg/day), CD-related antibiotics and/or immunomodulatory agents (azathioprine, 6-mercaptopurine, or methotrexate). Corticosteroid dose was tapered after Week 10.
All subjects received three intravenous induction doses of 5 mg/kg infliximab-dyyb at Week 0, 2 and 6. In order to be ranomized to treatment in CD Trial I, subjects had to be in clinical response at Week 10. Clinical response was defined as a decrease from baseline in CDAI of at least 100 points (i.e., CDAI-100 responders).
A total of 323 subjects were randomized at Week 10 in a double-blind fashion (2:1) to ZYMFENTRA 120 mg as a subcutaneous injection or placebo every 2 weeks.
At the time of randomization into thd double-blind phase (Week 10), 61% were receiving aminosalicylates, 40 % were receiving oral corticosteroids, and 32% were receiving immunomodulators including azathioprine, 6-mercaptopurine, or methotrexate.
A total of 11% of randomized subjects had prior exposure to biological products.
Subjects in the double-blind phase had a mean age of 35 years (range 18 to 75 years); 40% were female; and 91% identified as White, 4% identified as American Indian or Alaska Native, 4% identified as Asian, 0.3% as Black or African American, and 1% identified as another racial group.
The co-primary endopoints were clinical remission (based on CDAI) and endoscopic response at Week 54. Secondary endpoints included endoscopic remission, and corticosteroid-free remission at Week 54 (see Table 4).
Table 4: Proportion of Subjects with Crohn's Disease Meeting Efficacy Endpoints at Week 54 in CD Trial I
Endpoint
ZYMFENTRA
Placebo
Treatment Differencea
and 95% CI
Clinical Remission (Based on CDAI)bat Week 54
Total population
N=216
63%
N=107
30%
35%c
(24, 45)
No prior biological product expousre N=191
62%
N=98
31%
Prior biological product exposure N=25
72%
N=9
22%
Endoscopic responsed at Week 54
Total population N=216
50%
N=107
87%
34%c
(23, 43)
No prior biological product exposure N=191
51%
N=98
17%
Prior biological product exposure N=25
48%
N=9
22%
Enoscopic Remissione at Week 54
Total population
N=216
35%
N=107
10%
25%c
(16, 33)
No prior biological product exposure
N=191
35%
N=98
10%
Prior biological product exposure
N=25
36%
N=9
11%
Corticosteroid-free Remissionf at Week 54
Total population N=92
40%
N=43
21%
19%g No prior biological product expousre N=81
35%
N=40
23%
Prior biological product exposure N=11
82%
N=3
0%
CI=confidence internal, CDAI=Crohn's Disease Activity Index, JAK=Janus kinase
a Treatment difference (adjusted for stratification factors of previous exposure to biological product, use of treatment with oral corticosteroids at Week 0, and clinical remission status at Week 10).
b Clinical remission (based on CDAI) is defined as an absolute CDAI score of <150 points.
c p < 0.0001.
d Endoscopic response is defined as a >50% decrease in SES-CD from the baseline value.
e Endoscopic remission is defined as an absolute SES-CD score of ≤4 with no sub-score of >1.
f Corticosteroid-free remission is defined as being in clinical remission in additio to not receiving any corticosteroid for at least 8 weeks prior to Week 54, among the subjects who used oral corticosteroids at baseline.
g p < 0.05
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ZYMFENTRA (Infliximab-dyyb) injection for subcutaneous use is supplied as a sterile, preservative-free, clear to opalescent, colorless to pale brown solution in a single-use prefilled syringe, prefilled syringe with needle guard or prefilled pen. The syringe is fitted with a needle shield which are not made with natural rubber latex or any derivatives from natural rubber latex in any ingredient.
Prefilled Syringe
Each prefilled syringe is equipped with a 29 gauge fixed 1/2 inch needle with a rigid needle shield and a plunger stopper. The following configurations are available:
- One prefilled syringe (120 mg/mL solution) and one dose tray with two alcohol pads. (NDC: 72606-025-05)
- Two prefilled syringes (120 mg/mL solution) and two dose trays with two alcohol pads. (NDC: 72606-025-06)
- Four prefilled syringes (120 mg/mL solution) and four dose trays with four alcohol pads. (NDC: 72606-025-07)
- Six prefilled syringes (120 mg/mL solution) and six dose trays with six alcohol pads. (NDC: 72606-025-08)
Prefilled Syringe with Needle Guard
Each prefilled syringe is equipped with a 29 gauge fixed 1/2 inch needle with a rigid needle shield and an automatic needle guard, and a plunger stopper. The following configurations are available:
- One prefilled syringe with needle guard (120 mg/mL solution) and one does tray with two alcohol pads. (NDC: 72606-025-09)
- Two prefilled syringes with needle guard (120 mg/mL solution) and two dose trays with two alcohol pads. (NDC: 72606-025-10)
- Four prefilled syringes with needle guard (120 mg/mL solution) and four dose trays with four alcohol pads. (NDC: 72606-025-11)
- Six prefilled syringes with needle guard (120 mg/mL solution) and six dose trays with six alcohol pads. (NDC: 72606-025-12)
Prefilled Pen
Each prefilled pen is equipped with a 27 gauge fixed 1/2 inch needle with a rigid needle shield and a plunger stopper. The following configurations are available:
- One prefilled pen (120 mg/mL solution) and one dose tray with two alcohol pads. (NDC: 72606-025-01)
- Two prefilled pens (120 mg/mL solution and two dose trays with two alcohol pads. (NDC: 72606-025-02)
- Four prefilled pens (120 mg/mL solution) and four dose trays with four alcohol pads. (NDC: 72606-025-03)
- Six prefilled pens (120 mg/mL solution) and six dose trays with six alcohol pads. (NDC: 72606-025-04)
Storage and Handling
Store in refrigerator at 2°C to 8°C (36°F to 46°F). DO NOT FREEZE. Keep the product in its outer carton until time of administration in order to protect from light.
If needed, the product may be stored at room temperature at 20°C to 25°C (68°F to 77°F) for up to 14 days with protection from light. Once the product has been stored at room temperature, it should not be placed back into the refrigerator. The product must be discarded if not used within the 14 days.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient or thier caregiver to read the FDA-Approved Patient Labeling (Medication Guide and Instructions for Use).
Patients or their caregivers should be advised of the potential benefits and risks of ZYMFENTRA. Healthcare providers should instruct their patients or their caregivers to read the Medication Guide before starting ZYMFENTRA therapy and to reread it each time they receive an injection.
Infections
Inform patients that ZYMFENTRA increases the risk for developing serious infections. Instruct patients of the importance of contacting their healthcare provider if they develop any symptoms of an infection, including tuberculosis, invasive fungal infections, and reactivation of hepatitis B virus infections [see Warnings and Precautions (5.1, 5.3)].
Malignancies
Malignancies have been reported among children, adolescents and young adults who received treatment with TNF blockers. Patients should be counseled about the risk of lymphoma and other malignancies while receiving ZYMFENTRA [see Warnings and Precautions (5.2)].
Hepatotoxicity
Instruct patients to seek medical attention if they develop signs or symptoms of hepatotoxicity (e.g., jaundice) [see Warnings and Precautions (5.4)].
Congestive Heart Failure
Instruct patients to seek medical attention and consult their prescriber if they develop signs or symptoms of heart failure [see Warnings and Precautions (5.5)].
Hematologic Reactions
Instruct patients to seek immediate medical attention if they develop signs and symptoms suggestive of blood dyscrasias or infection (e.g., persistent fever) while on ZYMFENTRA [seeWarnings and Precautions (5.6)].
Hypersensitivity
Advise patients to seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions [see Warnings and Precautions (5.7)].
Neurologic Reactions
Advise patients to seek medical attention if they develop signs or symptoms of neurologic reactions [seeWarnings and Precautions (5.8)].
Live Vaccines/Therapeutic Infectious Agents
Instruct ZYMFENTRA-treated patients to avoid receiving live vaccines or therapeutic infectious agents [seeWarnings and Precautions (5.12)]
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 02/2024 MEDICATION GUIDE
ZYMFENTRATM (Zim fen' trah)
(infliximab-dyyb)
injection, for subcutaneous useRead the Medication Guide that comes with ZYMFENTRA before you receive the first treatment, and before each time you receive a treatment of ZYMFENTRA. This Medication Guide does not take the place of talking with your doctor about your medical condition or treatment. What is the most important information I should know about ZYMFENTRA?
ZYMFENTRA may cause serious side effects, including:
1. Risk of infection
ZYMFENTRA is a medicine that affects your immune system. ZYMFENTRA can lower the ability of your immune system to fight infections. Serious infections have happened in patients receiving ZYMFENTRA. These infections include tuberculosis (TB) and infections caused by viruses, fungi, or bacteria that have spread throughout the body. Some patients have died from these infections.- Your doctor should test you for TB before starting ZYMFENTRA.
- Your doctor should monitor you closely for signs and symptoms of TB during treatment with ZYMFENTRA.
- think you have an infection. You should not start receiving ZYMFENTRA if you have any kind of infection.
- are being treated for an infection.
- have signs of an infection, such as a fever, cough, flu-like symptoms.
- have any open cuts or sores on your body.
- get a lot of infections or have infections that keep coming back.
- have diabetes or an immune system problem as people with these conditions have a higher chance for getting infections.
- have TB, or have been in close contact with someone with TB.
- live or have lived in certain parts of the country (such as the Ohio and Mississippi River valleys) where there is an increased risk for getting certain kinds of fungal infections (histoplasmosis, coccidioidomycosis, or blastomycosis); these infections may develop or become more severe if you receive ZYMFENTRA. If you do not know if you have lived in an area where histoplasmosis, coccidioidomycosis, or blastomycosis is common, ask your doctor.
- have or have had hepatitis B.
- use the medicines KINERET (anakinra), ORENCIA (abatacept), or other medicines called biologics used to treat the same conditions as ZYMFENTRA.
After starting ZYMFENTRA, if you have an infection, any sign of an infection including a fever, cough, flu-like symptoms, or have open cuts or sores on your body, call your doctor right away. ZYMFENTRA can make you more likely to get infections or make any infection that you have worse.
2. Risk of Cancer- There have been cases of unusual cancers in children and teenage patients using tumor necrosis factor (TNF) blocker medicines, such as ZYMFENTRA.
- For people receiving TNF blocker medicines, including ZYMFENTRA, the chances of getting lymphoma or other cancers may increase.
- Some people receiving TNF blockers, including ZYMFENTRA, developed a rare type of cancer called hepatosplenic T-cell lymphoma. This type of cancer often results in death. Most of these people were male teenagers or young men. Also, most people were being treated for Crohn's disease or ulcerative colitis with a TNF blocker and another medicine called azathioprine or 6-mercaptopurine.
- People who have been treated for Crohn's disease and ulcerative colitis, for a long time may be more likely to develop lymphoma. This is especially true for people with very active disease.
- Some people treated with infliximab products, such as ZYMFENTRA, have developed certain kinds of skin cancer. If any changes in the appearance of your skin or growths on your skin occur during or after your treatment with ZYMFENTRA, tell your doctor.
- Patients with Chronic Obstructive Pulmonary Disease (COPD), a specific type of lung disease, may have an increased risk for getting cancer while being treated with ZYMFENTRA.
- Tell your doctor if you have ever had any type of cancer. Discuss with your doctor any need to adjust medicines you may be taking.
What is ZYMFENTRA?
ZYMFENTRA is a prescription medicine used as an injection uner the skin (subcutaneous injection) by adults for the maintenance treatment of:- moderately to severely active ulcerative colitis following treatment with an infliximab product given by intravenous infusion (IV).
- moderately to severely active Crohn's disease following treatment with an infliximab product given by intravenous infusion (IV).
Do not take ZYMFNETRA if you:
- have had an allergic reaction to ZYMFENTRA, other infliximab products, any murine proteins or any of the ingredients in ZYMFENTRA. See the end of this Medication Guide for a complete list of ingredients in ZYMFENTRA.
Before you receive ZYMFENTRA, tell your doctor all of your medical conditions, including if you:
- have an infection (see "What is the most important information I should know about ZYMFENTRA?").
- have other liver problems including liver failure.
- have heart failure or other heart conditions. If you have heart failure, it may get worse while you receive ZYMFENTRA.
- have or have had any type of cancer.
- have COPD (Chronic Obstructive Pulmonary Disease), a specific type of lung disease. Patients with COPD may have an increased risk of getting cancer while receiving ZYMFENTRA.
- have or have had a condition that affects your nervous system such as:
- multiple sclerosis, or Guillain-Barré syndrome, or
- if you experience any numbness or tingling, or
- if you have had a seizure.
- have recently received or are scheduled to receive a vaccine. Adults receiving ZYMFENTRA should not receive live vaccines (for example, the Bacille Calmette-Guérin [BCG] vaccine) or treatment with a weakened bacteria (such as BCG for bladder cancer). Adults should have all of their vaccines brought up to date before starting treatment with ZYMFENTRA.
- are pregnant or plan to become pregnant, are breastfeeding or plan to breastfeed. You and your doctor should decide if you should receive ZYMFENTRA while you are pregnant or breastfeeding.
If you have a baby and you were receiving ZYMFENTRA during your pregnancy, it is important to tell your baby's doctor and other healthcare professionals about your ZYMFENTRA use so they can decide when your baby should receive any vaccine. Certain vaccinations can cause infections.
If you received ZYMFENTRA while you were pregnant, your baby may be at higher risk for getting an infection. If your baby receives a live vaccine within 6 months after birth, your baby may develop infections with serious complications that can lead to death. This includes live vaccines such as the BCG, rotavirus, or any other live vaccines. For other types of vaccines, talk with your doctor.How should I receive ZYMFENTRA?
- Use ZYMFENTRA exactly as your doctor tells you to.
- If your healthcare provider decides that you or your cargiver can give your injections of ZYMFENTRA at home, you or your caregiver should be shown the right way to prepare and inject ZYMFENTRA.
- Do not try to inject ZYMFENTRA yourself until you or your caregiver have been shown how to inject ZYMFENTRA by your healthcare provider.
- ZYMFENTRA is injected under your skin (subcutaneously) 1 time every two weeks.
- Inject ZYMFENTRA under the skin (subcutaneous injection), in your upper arms, stomach area (abdomen), or upper legs (thighs).
- Do no give an injection in an area of the skin that is tender, bruised, red or hard.
- Use a different injection site each time you use ZYMFENTRA.
- If you are not able to inject ZYMFENTRA at your regular scheduled time or you miss a dose of ZYMFENTRA, inject the dose as soon as possible. Then, inject your next dose every two weeks thereafter. If you are not sure when to inject ZYMFENTRA, call your healthcare provider.
- If you inject more than prescribed, call your doctor right away.
- Be sure to keep all of your scheduled follow-up appointments.
Read the detailed Instructions for Use at the end of this Medication Guide for insructions about how to prepare and inject a dose of ZYMFENTRA, and how to properly throw away (dispose of) used needles and syringes. The syringe and needle must never be re-used. After the rubber stopper is puntured, ZYMFENTRA can become contaiminated by harmful bacteria which could cause an infection if re-used. Therefore, throw away any unused portion of ZYMFENTRA.
What should I avoid while taking ZYMFENTRA?
Do not take ZYMFENTRA together with other medicines medicines called biologics that are used to treat the same conditions as ZYMFENTRA.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. These include any other medicines to treat Crohn's disease, or ulcerative colitis.
Know the medicines you take. Keep a list of your medicines and show them to your doctor and pharmacist when you get a new medicine.What are the possible side effects of ZYMFENTRA?
ZYMFENTRA can cause serious side effects, including (see "What is the most important information I should know about ZYMFENTRA?"):
Serious Infections- Some patients, especially those 65 years and older have had serious infections while receiving infliximab products, such as ZYMFENTRA. These serious infections include TB and infections caused by viruses, fungi, or bacteria that have spread throughout the body or cause infections in certain areas (such as skin). Some patients die from these infections. If you get an infection while receiving treatment with ZYMFENTRA your doctor will treat your infection and may need to stop your ZYMFENTRA treatment.
- Tell your doctor right away if you have any of the following signs of an infection while receiving or after receiving ZYMFENTRA:
- a fever
- feel very tired
- have a cough
- have flu-like symptoms
- warm, red, or painful skin
- Your doctor will examine you for TB and perform a test to see if you have TB. If your doctor feels that you are at risk for TB, you may be treated with medicine for TB before you begin treatment with ZYMFENTRA and during treatment with ZYMFENTRA.
- Even if your TB test is negative, your doctor should carefully monitor you for TB infections while you are receiving ZYMFENTRA. Patients who had a negative TB skin test before receiving infliximab products may develop active TB after receiving infliximab products.
- If you are a chronic carrier of the hepatitis B virus, the virus can become active while you are being treated with ZYMFENTRA. In some cases, patients have died as a result of hepatitis B virus being reactivated. Your doctor should do a blood test for hepatitis B virus before you start treatment with ZYMFENTRA, while you are being treated and for several months after you finish treatment. Tell your doctor if you have any of the following symptoms:
- feel unwell
- poor appetite
- tiredness (fatigue)
- fever, skin rash, or joint pain
Liver Injury
Some patients receiving infliximab products have developed serious liver problems. Tell your doctor if you have:
- jaundice (skin and eyes turning yellow)
- dark, brwon-colored urine
- pain on the right side of your stomach area (right-sided abdominal pain)
- fever
- extreme tiredness (sevre fatigue)
Heart Failure
If you have a heart problem called congestive heart failure, your doctor should check you closely while you are receiving ZYMFENTRA. Your congestive heart failure may get worse while you are receiving ZYMFENTRA. Be sure to tell your doctor of any new or worse symptoms including:
- shortness of breath
- swelling of ankles or feet
- sudden weight gain
Treatment with ZYMFENTRA may need to be stopped if you get new or worse congestive heart failure.
Blood Probelms
In some patiens receiving infliximab products, the body may not make enough of the blood cells tha help fight infections or help stop bleeding. Tell your doctor if you:
- have a fever that does not go away
- bruise or bleed very easily
- look very pale
Allergic Reactions
Some patients have had allergic reactions to infliximab products. Some of these reactions were severe. These reactions can happen while you are getting your ZYMFENTRA treatment or shortly afterward. Your doctor may need to stop or pause your treatment with ZYMFENTRA and may give you medicines to treat the allergic reaction. Signs of an allergic reactio can include:- hives (red, raised, itchy patches of skin)
- difficulty breathing
- chest pain
- high or low blood pressure
- fever
- chills
Some patients treated with infliximab products have had delayed allergic reactions. Tell your doctor right away if you have any of these signs of delayed reaction to ZYMFENTRA:
- fever
- rash
- headache
- sore throat
- muscle or joint pain
- swelling of the face and hands
- difficulty swallowing
Nervous System Disorders
Some patients receiving infliximab products have developed problems with their nervous system. Tell your doctor if you have:
- changes in your vision
- numbness or tingling in any part of your body
- seizures
- weakness in your arms or legs
Some patients have experienced a stroke within approximately 24 hours of their infusion with infliximab products. Tell your doctor right away if you have symptoms of a stroke which may include: numbness or weakness of the face, arm, or leg, especially on one side of the body; sudden confusion, trouble speaking or understanding; sudden trouble seeing in one or both eyes, sudden trouble walking, dizziness, loss of balance or coordination or a sudden, severe headache.
Lupus-like Syndrome
Some patient have developed symptoms that are like the symptoms of Lupus. If you develop any of the following symptoms, your doctor may decide to stop your treatment with ZYMFENTRA:
- chest disomfort or painthat does not go away
- shortness of breath
- joint pain
- rash on the cheeks or arms that gets worse in the sun
The most common side effects of ZYMFENTRA include:
- COVID-19
- respiratory infections, such as sinus infections and sore throat
- injection site reactions
- headache
- abdominal pain
- abnormal liver enzymes
- joint pain
- diarrhea
- high blood pressure
- urinary tract infections
- dizziness
Tell your doctor about any side effect that bothers you or does not go away.
These are not all of the side effects with ZYMFENTRA. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store ZYMFENTRA?
- Store ZYMFENTRA prefilled syringes and prefilled pens in a refrigerator between 36°F to 46°F (2°C to 8°C).
- If needed, ZYMFENTRA prefilled syringes and prefilled pens may be stored at room temperature between 68°F to 77°F (20°C to 25°C) for up to 14 days with protection from light.
- When ZYMFENTRA prefilled syringes and prefilled pens have reached room temperature, do not put ZYMFENTRA back in the refrigerator. ZYMFENTRA must be thrown away (discarded) if not used within the 14 days.
- Do not freeze ZYMFENTRA.
- Do not shake ZYMFENTRA.
- Keep ZYMFENTRA in the original carton until ready to use to protect it from light.
General information about ZYMFENTRA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
You can ask your doctor or pharmacist for information about ZYMFENTRA that is written for health professionals.
Do not use ZYMFENTRA for a condition for which it was not prescribed. Do not give ZYMFENTRA to other people, even if they have the same symptoms that you have.
For more information call 1-888-804-3433.What are the ingredients in ZYMFENTRA?
The active ingredient is infliximab-dyyb.
The inactive ingredients in ZYMFENTRA include: acetic acid, polysorbate 80, sodium acetate, and sorbitol in water for injections. No preservatives are present.Manufactured by: CELLTRION, Inc. 23, Academy-ro, Yeonsu-gu, Incheon, 22014, Republic of Korea
U.S. License No. 1996 ©CELLTRION, Inc.
Distributed by CELLTRION USA, Inc. One Evertrust Plaza Suite 1207, Jersey City, New Jersey, 07302, USA
For more information call 1-888-804-3433 -
INSTRUCTIONS FOR USE
ZYMFENTRA (Zim fen' trah)
(infliximab-dyyb)
Injection for subcutaneous use only
Single-Dose Prefilled Pen120 mg/mL Rx Only
Read and follow the Instruction for Use that come with your ZYMFENTRA Prefilled Pen before you start using it and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Figure A: Parts of ZYMFENTRA Prefilled Pen
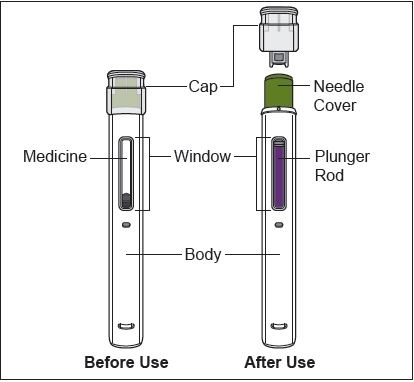
Important Information
- Use the Prefilled Pen only if your healthcare provider has trained you on the right way to prepare for and to give an injection.
- Ask your healthcare provider how often you will need to give an injection.
- Rotate the injection site each time you give an injection. Each new injection site should be at least 1.2 inches away from the previous injection site.
- Do not shake the Prefilled Pen at any time.
- Do not remove the Cap until you are ready to inject.
- Do not share the Prefilled Pen with anyone.
How to store the Prefilled Pen
-
Store the Prefilled Pen in a refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the Prefilled Pen in the original carton until use to protect it from light.
- Do not use the Prefilled Pen that has been left in direct sunlight.
- Do not freeze the Prefilled Pen. If the Prefilled Pen has been frozen, do not use the Prefilled Pen even if it is thawed.
- Do not warm the Prefilled Pen using heat sources such as hot water or a microwave. Let the Prefilled Pen naturally warm at room temperature between 68°F to 77°F (20°C to 25°C) for 30 minutes before giving an injection.
- When the Prefilled Pen has reached room temperature, do not put it back in the refrigerator. The Prefilled Pen must be thrown away (discarded) if not used within the 14 days.
- Keep the Prefilled Pen and all medicines out of the reach of children.
Prepare for the Injection
1. Gather the supplies for the injection.
1a. Prepare a clean, flat surface, such as a table or countertop, in a well-lit area.
1b. Remove 1 Prefilled Pen from the carton stored in your refrigerator.
Note: Return the carton with any unused Prefilled Pens to refrigerator immediately after taking out one Prefilled Pen.
1c. Make sure you have the following supplies:
- Prefilled Pen
- 1 Alcohol swab
Not included in the carton:
- 1 Cotton ball or gauze
- 1 Adhesive bandage
- FDA-cleared sharps disposal container2. Inspect the Prefilled Pen.
2a. Make sure you have the correct medicine (ZYMFENTRA).
2b. Check the expiration (EXP) date on the label of the Prefilled Pen (See Figure B).
2c. Look at the Prefilled Pen and make sure it is not cracked or damaged.
-
Do not use the Prefilled Pen if:
- it is cracked or damaged.
- the expiration (EXP) date has passed.
 Figure B
Figure B
3. Inspect the Medicine.
3a. Look through the Window and make sure that the liquid is clear, colorless to pale brown, and free of particles (see Figure C).
- Do not use the Prefilled Pen if the liquid is the discolored (yellow or dark brown), cloudy, or contains particles in it.
- You may see air bubbles in the liquid. This is normal.
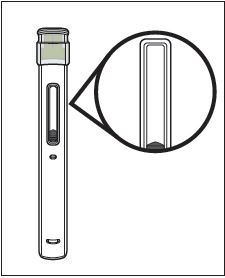 Figure C
Figure C
4. Wait 30 minutes.
4a. Leave the Prefilled Pen at room temperature 68°F to 77°F (20°C to 25°C) for 30 minutes to allow it to warm up (see Figure D).
- Do not warm the Prefilled Pen using heat sources such as hot water or a microwave.
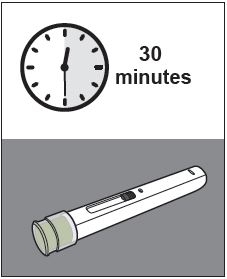
Figure D
5. Choose an injection site (see Figure E).
5a. You may inject into:
- the front of your thighs.
- the stomach area (abdomen) except for the 2 inches around the belly button (navel).
- the outer area of the upper arm if you are a caregiver.
- Do not inject into skin that is within 2 inches of your belly button (navel), or is red, hard, tender, damaged, bruised, or scarred.
- Do not inject through your clothes.
5b. Rotate the injection site each time you give an injection.
- Do not inject the same injection site each time you give an injection.
- Each new injection site should be at least 1.2 inches away from the injection site you used before.
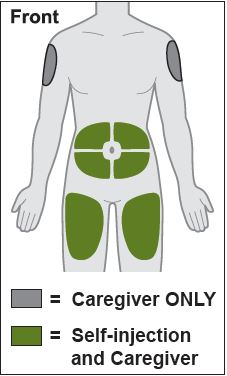
Figure E
6. Wash your han
6a. Wash your hands with soap and water and dry them thoroughly (see Figure F).
Figure F
7. Clean the injection site.
7a. Clean the injection site with an alcohol swab using a circular motion (see Figure G).
7b. Let the skin dry before injecting.- Do not blow on or touch the injection site again before giving the injection.
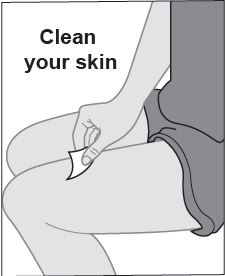
Figure G
Give the injection
8. Remove the Cap.
8a. Hold the Prefilled Pen by the injector body with the Cap on top using one hand. Gently pull the Cap straight off with the other hand.
- Do not recap the Prefilled Pen.
- Do not remove the Cap until you are ready to inject.
- Do not touch the Needle or Needle Cover. Doing so may result in a needle stick injury.
8b. Dispose of the Cap in an FDA cleared sharps container (see Step 12 and Figure H).
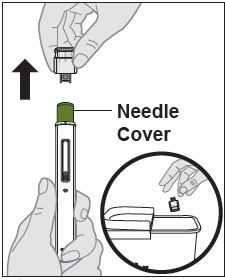
Figure H
9. Place the Prefilled Pen on the injection site.
9a. Hold the Prefilled Pen so that you can see the Window.
9b. Without pinching or stretching the skin, place the Prefilled Pen over the injection site at a 90-degree angle (see Figure I).
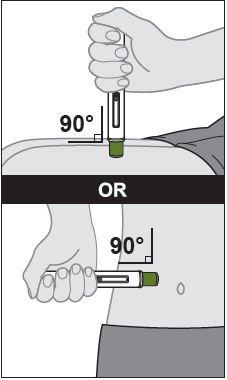
Figure I
10.Give the injection (see Figure J).
10a. Press the Prefilled Pen firmly against the skin.
- When the injection starts you will hear the first loud "click" and the purple Plunger Rod will begin to fill the Window.
10b. Keep holding the Prefilled Pen firmly against the skin and listen for the second loud "click." This can take up to 10 seconds.
- Do not change the position of the Prefilled Pen after the injection has started.
10c. After you hear the second loud "click" continue to keep holding the Prefilled Pen firmly against the skin and count slowly to 5 to make sure you inject the full dose.
10d. Look at the Prefilled Pen and make sure that the purple Plunger Rod is filling the Window completely.
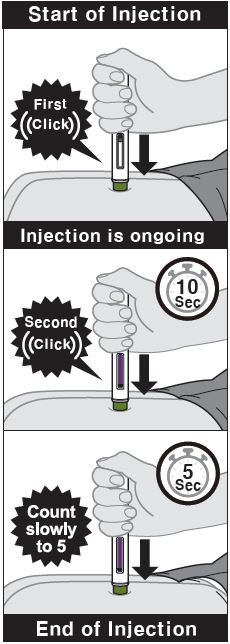
Figure J
11. Remove the Prefilled Pen from your skin.
11a. Remove the Prefilled Pen from your skin (see Figure K).
- After you remove the Prefilled Pen from the injection site, the needle will be automatically covered (see Figure L).
- If the Window has not turned completely purple or if the medicine is still injecting, this means you have not received a full dose. Call your healthcare provider immediately.
- You may see grey stopper in the Window. This is normal.
- Some bleeding may occur.
- Do not reuse the Prefilled Pen.
- Do not rub the injection site
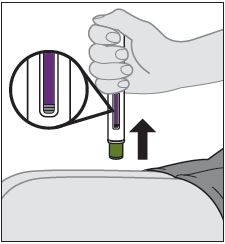
Figure K
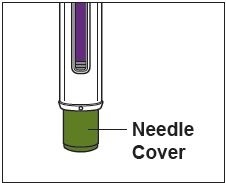
Figure L
After the injection
12. Throw away (dispose of) the Prefilled Pen.
12a. Put the used Prefilled Pen in an FDA-cleared sharps disposal container right away after use (see Figure M).
-
Do not throw away (dispose of) the Prefilled Pen in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container. - When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of it. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
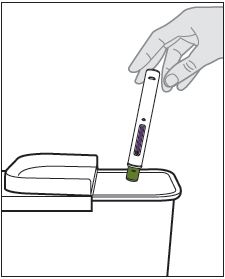
Figure M
13. Care for the injection site.
13a. Treat the injection site by gently pressing, not rubbing, a cotton ball or gauze to the site and apply an adhesive bandage, if necessary.
Manufactured by: CELLTRION, Inc., 23, Academy-ro, Yeonsu-gu, Incheon, 22014, Republic of Korea
US License Number 1996Distributed by: CELLTRION USA, Inc., 1 Evertrust Plaza Suite 1207, Jersey City, NJ 07302
-
INSTRUCTIONS FOR USE
ZYMFENTRA (Zim fen' trah)
(infliximab-dyyb)
Injection for subcutaneous use only
Single-Dose Prefilled Syringe
120 mg/mL
Rx Only
For subcutaneous use only
Read and follow Instructions for Use that come with your ZYMFENTRA Prefilled Syringe before using it and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Figure A: Parts of ZYMFENTRA Prefilled Syringe
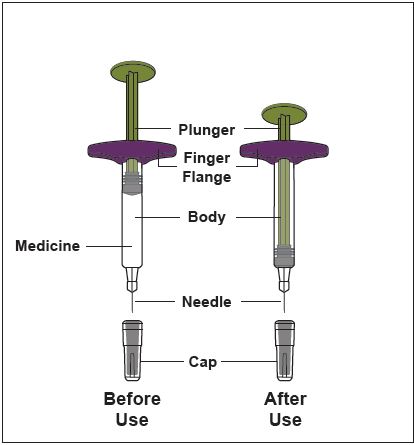
Caution! Do not remove the Cap until you are ready to inject. When you remove the Cap, do not recap the Prefilled Syringe.
Important Information
- Use the Prefilled Syringe only if your healthcare provider has trained you on the right way to prepare for and to give an injection.
- Ask your healthcare provider how often you will need to give an injection.
- Rotate the injection site each time you give an injection. Each new injection site should be at least 1.2 inches away from the previous injection site.
- Do not use Prefilled Syringe if it has been dropped or is visibly damaged.
- Do not reuse the Prefilled Syringe.
- Do not shake the Prefilled Syringe at any time.
- Do not share the Prefilled Syringe with anyone.
- Do not pull back on the plunger rod at any time.
- Only use each Prefilled Syringe for one injection.
How to store the Prefilled Syringe
- Store the Prefilled Syringe in a refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the Prefilled Syringe in the original carton to protect it from light.
- Do not use the Prefilled Syringe that has been left in direct sunlight.
- Do not freeze the Prefilled Syringe. If the Prefilled Pen has been frozen, do not use the Prefilled Syringe even if it is thawed.
- Do not warm the Prefilled Syringe using heat sources such as hot water or a microwave. Let the Prefilled Syringe naturally warm at room temperature between 68°F to 77°F (20°C to 25°C) for 30 minutes before giving an injection.
- When the Prefilled Syringe has reached room temperature, do not put it back in the refrigerator. The Prefilled Syringe must be thrown away (discarded) if not used within the 14 days.
- Keep the Prefilled Syringe and all medicines out of the reach of children.
Prepare for the Injection
1. Gather the supplies for the injection.
1a. Prepare a clean, flat surface, such as a table or countertop, in a well-lit area.
1b. Remove 1 Prefilled Syringe from the carton by holding the middle of the Prefilled Syringe Body.- Do not touch the plunger rod.
Note: Return the carton with any unused Prefilled Syringe to the refrigerator immediately after taking out one Prefilled Syringe.
1c. Make sure you have the following supplies:
- Prefilled Syringe
- Alcohol swab
Not included in the carton:
- Cotton ball or gauze
- Adhesive bandage
- FDA-cleared sharps disposal container2. Inspect the Prefilled Syringe.
2a. Make sure you have the correct medicine (ZYMFENTRA).
2b. Check the expiration (EXP) date on label of the Prefilled Syringe (See Figure B).
2c. Look at the Prefilled Syringe and make sure it is not cracked or damaged.
-
Do not use the Prefilled Syringe if:
- it is cracked or damaged.
- the expiration (EXP) date has passed.
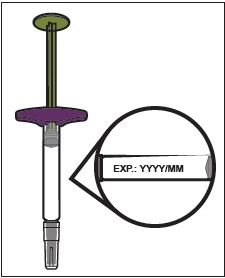
Figure B
3. Inspect the Medicine.
3a. Look at the Prefilled Syringe Body and check the liquid is clear and colorless to pale brown (see Figure C).
- Do not use the Prefilled Syringe if the liquid is wrong color, cloudy, or contains particles in it.
- You may see air bubbles in the liquid. This is normal.
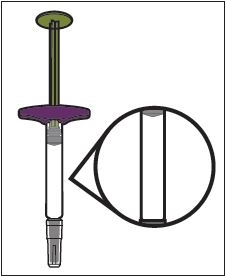 Figure C
Figure C
4. Wait 30 minutes.
4a. Leave the Prefilled Syringe at room temperature 68°F to 77°F (20°C to 25°C) for 30 minutes to allow it to warm up (see Figure D).
- Do not warm the Prefilled Syringe using heat sources such as hot water or a microwave.
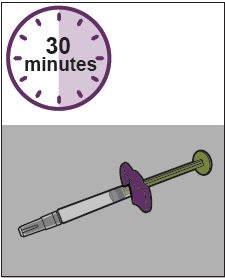
Figure D
5. Choose an injection site (see Figure E).
5a. You may inject into:
- the front of your thighs.
- the stomach area (abdomen) except for the 2 inches around the belly button (navel).
- the outer area of the upper arm (caregiver only).
- Do not inject into skin that is within 2 inches of your belly button (navel), or is red, hard, tender, damaged, bruised, or scarred.
- Do not inject through your clothes.
5b. Rotate the injection site each time you give an injection.
- Do not inject the same injection site each time you give an injection.
- Each new injection site should be at least 1.2 inches away from the previous injection site.
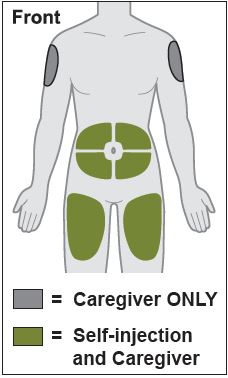
Figure E
6. Wash your hands.
6a. Wash your hands with soap and water and dry them thoroughly (see Figure F).
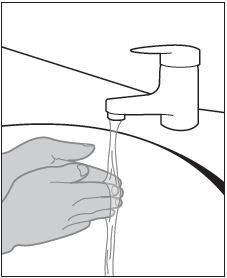
Figure F
7. Clean the injection site.
7a. Clean the injection site with an alcohol swab using a circular motion (see Figure G).
7b. Let the skin dry before injecting.- Do not blow on or touch the injection site again before giving the injection.
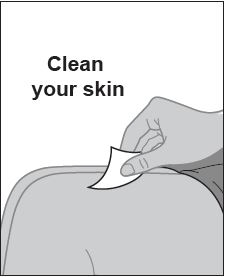
Figure G
Give the injection
8. Remove the Cap.
8a. Hold the Prefilled Syringe by the injector body with the Cap on top using one hand. Gently pull the Cap straight off with the other hand.
- Do not pull back on plunger rod at any time.
- Do not remove the Cap until you are ready to inject.
- Do not try to put the Cap back onto the Prefilled Syringe.
- Do not touch the Needle.
8b. Throw away (dispose of) the Cap in an FDA cleared sharps container (see Step 12 and Figure H).
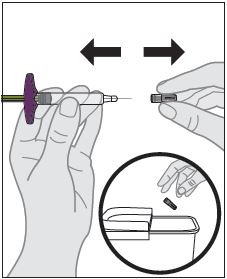
Figure H
9. Insert the Prefilled Syringe into the injection site.
9a. Hold the Prefilled Syringe by its body in one hand between your thumb and index finger.
9b. Gently pinch a fold of skin at the injection site with one hand.
9c. With a quick and “dart-like” motion, insert the Needle completely into the fold of the skin at a 45-defree angle (see Figure I).
- Do not change the position of the Prefilled Syringe after the injection has started.
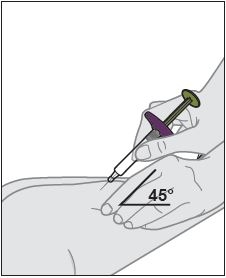
Figure I
10.Give the injection.
10a. After the Needle is inserted, release the pinched skin.
10b. Push the Plunger down slowly and as far as it will go until the Prefilled Syringe is empty (See Figure J).
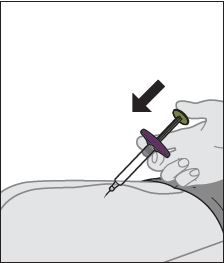
Figure J
11. Remove the Prefilled Syringe from the injection site.
11a. Remove the Needle from your skin at the same angle it was inserted (see Figure K).
- Do not reuse the Prefilled Syringe.
- Do not try to put the needle cover back on the Prefilled Syringe.
- Do not touch the Needle.
- Some bleeding may occur.
- Do not rub the injection site.
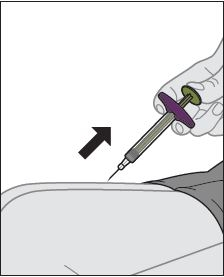
Figure K
After the injection
12. Throw away (dispose of) the Prefilled Syringe.
12a. Put the used Prefilled Syringe in an FDA-cleared sharps disposal container right away after use (see Figure L).
- Do not try to put the Cap back onto the Prefilled Syringe.
- Do not throw away (dispose of) the Prefilled Syringe in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- able to close with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container. - When your sharps disposal container is almost full, you will need to follow your community guidelines for right way to disposal of it. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
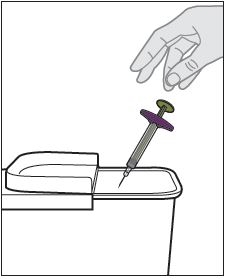
Figure L
13. Care for the injection site.
13a. Treat the injection site by gently pressing a cotton ball or gauze to the site and apply an adhesive bandage, if necessary.
Manufactured by: CELLTRION, Inc., 23, Academy-ro, Yeonsu-gu, Incheon, 22014, Republic of Korea
US License Number 1996Distributed by: CELLTRION USA, Inc., 1 Evertrust Plaza Suite 1207, Jersey City, NJ 07302
-
INSTRUCTIONS FOR USE
ZYMFENTRA (Zim fen' trah)
(infliximab-dyyb)
Injection for subcutaneous use only
Single-Dose Prefilled Syringe with Needle Guard
120 mg/mL
Rx Only
Read and follow the Instructions for Use that come with your ZYMFENTRA Prefilled Syringe before you start using it and each time you get a refill. There may be new information. This information dose not take the place of talking to your healthcare provider about your medical condition or treatment.
Figure A: Parts of Prefilled Syringe
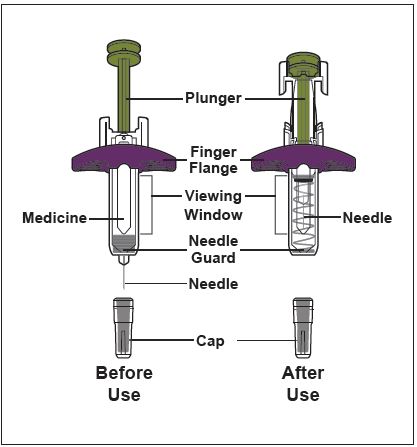
Caution! Do not remove the Cap until you are ready to inject. Once you remove the Cap, do not recap the Syringe.
Important Information
- Use the Prefilled Syringe only if your healthcare provider has trained you on the right way to prepare for and to give an injection.
- Ask your healthcare provider how often you will need to give an injection.
- Rotate the injection site each time you give an injection. Each new injection site should be at least 1.2 inches away from the previous injection site.
- Do not use Prefilled Syringe if it has been dropped or is visibly damaged.
- Do not reuse the Prefilled Syringe.
- Do not shake the Prefilled Syringe at any time.
- Do not share the Prefilled Syringe with anyone.
- Do not pull back on the plunger rod at any time.
- Only use each Prefilled Syringe for one injection.
How to store the Prefilled Syringe
- Store the Prefilled Syringe in a refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the Prefilled Syringe in the original carton until use to protect it from light.
- Do not use the Prefilled Syringe that has been left in direct sunlight.
- Do not freeze the Prefilled Syringe. If the Prefilled Pen has been frozen, do not use the Prefilled Syringe even if it is thawed.
- Do not warm the Prefilled Syringe using heat sources such as hot water or a microwave. Let the Prefilled Syringe naturally warm at room temperature between 68°F to 77°F (20°C to 25°C) for 30 minutes before giving an injection.
- When the Prefilled Syringe has reached room temperature, do not put it back in the refrigerator. The Prefilled Syringe must be thrown away (discarded) if not used within the 14 days.
- Keep the Prefilled Syringe and all medicines out of the reach of children.
Prepare for the Injection
1. Gather the supplies for the injection.
1a. Prepare a clean, flat surface, such as a table or countertop, in a well-lit area.
1b. Remove 1 Prefilled Syringe from the carton by holding the middle of the Prefilled Syringe Body.- Do not touch the plunger rod.
Note: Return the carton with any unused Prefilled Syringe to the refrigerator immediately after taking out one Prefilled Syringe.
1c. Make sure you have the following supplies:
- Prefilled Syringe
- Alcohol swab
Not included in the carton:
- Cotton ball or gauze
- Adhesive bandage
- FDA-cleared sharps disposal container2. Inspect the Prefilled Syringe.
2a. Make sure you have the correct medicine (ZYMFENTRA).
2b. Check the expiration (EXP) date on the label of the Prefilled Syringe (See Figure B).
2c. Look at the Prefilled Syringe and make sure it is not cracked or damaged.
-
Do not use the Prefilled Syringe if:
- it is cracked or damaged.
- the expiration (EXP) date has passed.
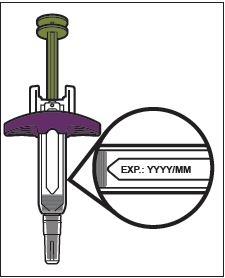
Figure B
3. Inspect the Medicine.
3a. Look through the Viewing Window and check that the liquid is clear and colorless to pale brown, and free of particles (see Figure C).
- Do not use the Prefilled Syringe if the liquid is wrong color, cloudy, or contains particles in it.
- You may see air bubbles in the liquid. This is normal.
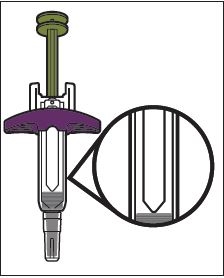
Figure C
4. Wait 30 minutes.
4a. Leave the Prefilled Syringe at room temperature 68°F to 77°F (20°C to 25°C) for 30 minutes to allow it to warm up (see Figure D).
- Do not warm the Prefilled Syringe using heat sources such as hot water or a microwave.
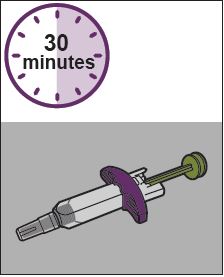
Figure D
5. Choose an injection site (see Figure E).
5a. You may inject into:
- the front of your thighs.
- the stomach area (abdomen) except for the 2 inches around the belly button (navel).
- the outer area of the upper arm (caregiver only).
- Do not inject into skin that is within 2 inches of your belly button (navel), or is red, hard, tender, damaged, bruised, or scarred.
- Do not inject through your clothes.
5b. Rotate the injection site each time you give an injection.
- Do not injection the same injection site each time you five an injection.
- Each new injection site should be at least 1.2 inches away from the previous injection site.
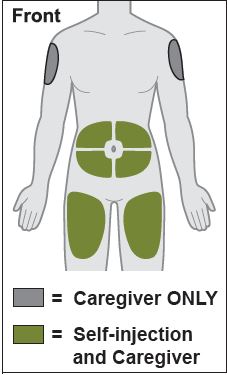
Figure E
6. Wash your hands.
6a. Wash your hands with soap and water and dry them thoroughly (see Figure F).
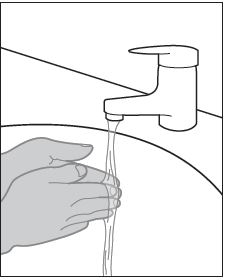
Figure F
7. Clean the injection site.
7a. Clean the injection site with an alcohol swab (see Figure G).
7b. Let the skin dry before injecting.- Do not blow on or touch the injection site again before giving the injection.
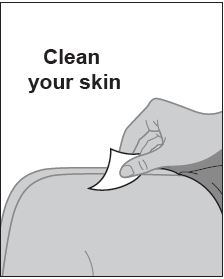
Figure G
Give the injection
8. Remove the Cap.
8a. Hold the Prefilled Syringe by the injector body with the Cap on top using one hand. Gently pull the Cap straight off with the other hand.
- Do not pull back on plunger rod at any time.
- Do not remove the Cap until you are ready to inject.
- Do not try to put the Cap back onto the Prefilled Syringe.
- Do not touch the Needle.
8b. Throw away (dispose of) the Cap in an FDA cleared sharps container (see Step 12 and Figure H).
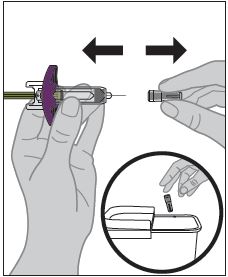
Figure H
9. Insert the Prefilled Syringe into the injection site.
9a. Hold the Prefilled Syringe by its body in one hand between your thumb and index finger.
9b. Gently pinch a fold of skin at the injection site with one hand.
9c. Witha quick and “dart-like” motion, insert the Needle completely into the fold of the skin at a 45-defree angle (see Figure I).
Ÿ Do not change the position of the Prefilled Syringe after the injection has started.
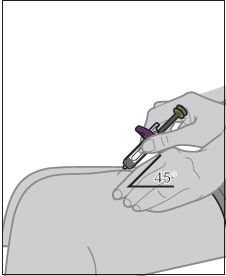
Figure I
10.Give the injection.
10a. After the Needle is inserted, release the pinched skin.
10b. Push the Plunger down slowly and as far as it will go until the Prefilled Syringe is empty (See Figure J).
Note: The Needle Guard will not activate unless all the Medicines is injected.
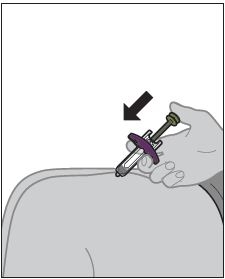
Figure J
11. Remove the Prefilled Syringe from the injection site.
11a. After the Prefilled Syringe is empty, slowly lift your thumb from the Plunger until Needle is completely covered by the Neeld Guard (see Figure K).
- Do not reuse the Prefilled Syringe.
- Do not touch the Needle.
- Some bleeding may occur.
- Do not rub the injection site.
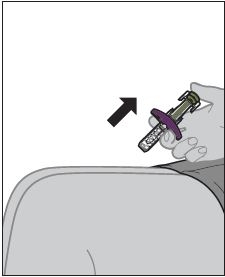
Figure K
After the injection
12. Throw away (dispose of) the Prefilled Syringe.
12a. Put the used Prefilled Syringe in an FDA-cleared sharps disposal container immediately after use (see Figure L).
- Do not try to put the Cap back onto the Prefilled Syringe.
- Do not throw away (dispose of) the Prefilled Syringe in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- able to closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to disposal of it. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
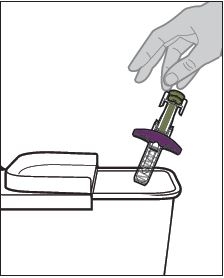
Figure L
13. Care for the injection site.
13a. Treat the injection site by gently pressing a cotton ball or gauze to the site and apply an adhesive bandage, if necessary.
Manufactured by: CELLTRION, Inc., 23, Academy-ro, Yeonsu-gu, Incheon, 22014, Republic of Korea
US License Number 1996Distributed by: CELLTRION USA, Inc., 1 Evertrust Plaza Suite 1207, Jersey City, NJ 07302
- PRINCIPAL DISPLAY PANEL - 120 mg/mL Prefilled Pen Label
-
PRINCIPAL DISPLAY PANEL - 120 mg/mL Prefilled Pen Carton
ZymfentraTM
infliximab-dyyb
Injection
120 mg/mLFOR SUBCUTANEOUS USE ONLY
ATTENTION: Disponse the enclosed Medication Guide to each patient.
NDC: 72606-025-01
Rx ony
1 prefilled pen + 2 alcohol preps
1 Single-Dose Prefilled Pen
CELLTRION USA, Inc.

- PRINCIPAL DISPLAY PANEL - 120 mg/mL Prefilled Syringe with Needle Guard Label
-
PRINCIPAL DISPLAY PANEL - 120 mg/mL Prefilled Syringe with Needle Guard Carton
ZymfentraTM
infliximab-dyyb
injection
120 mg/mLFOR SUBCUTANEOUS USE ONLY
ATTENTION: Dispense the enclosed Medication Guide to each patient.
NDC: 72606-025-09
1 prefilled syringe with needle guard
+ 2 alcohol preps1 Single-Dose Prefilled Syringe with Needle Guard
CELLTRION USA, Inc.
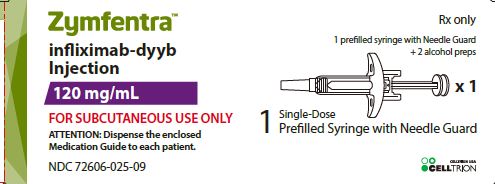
- PRINCIPAL DISPLAY PANEL - 120 mg/mL Prefilled Syringe Label
- PRINCIPAL DISPLAY PANEL - 120 mg/mL Prefilled Syringe Carton
-
INGREDIENTS AND APPEARANCE
ZYMFENTRA
infliximab-dyyb kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72606-025 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72606-025-01 1 in 1 CARTON 03/01/2024 1 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC: 72606-025-02 2 in 1 CARTON 03/01/2024 2 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 3 NDC: 72606-025-03 4 in 1 CARTON 03/01/2024 3 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 4 NDC: 72606-025-04 6 in 1 CARTON 03/01/2024 4 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 5 NDC: 72606-025-05 1 in 1 CARTON 03/01/2024 5 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 6 NDC: 72606-025-06 2 in 1 CARTON 03/01/2024 6 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 7 NDC: 72606-025-07 4 in 1 CARTON 03/01/2024 7 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 8 NDC: 72606-025-08 6 in 1 CARTON 03/01/2024 8 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 9 NDC: 72606-025-09 1 in 1 CARTON 03/01/2024 9 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 10 NDC: 72606-025-10 2 in 1 CARTON 03/01/2024 10 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 11 NDC: 72606-025-11 4 in 1 CARTON 03/01/2024 11 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 12 NDC: 72606-025-12 6 in 1 CARTON 03/01/2024 12 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE 1 mL Part 2 1 PACKET 1 mL Part 1 of 2 ZYMFENTRA
infliximab-dyyb injectionProduct Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INFLIXIMAB-DYYB (UNII: B72HH48FLU) (INFLIXIMAB - UNII:B72HH48FLU) INFLIXIMAB-DYYB 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) 0.19 mg in 1 mL SODIUM ACETATE (UNII: 4550K0SC9B) 0.56 mg in 1 mL SORBITOL (UNII: 506T60A25R) 45 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.5 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 TRAY 1 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761358 03/01/2024 Part 2 of 2 ALCOHOL
isopropyl alcohol swabProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.70 mL in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 10/31/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761358 03/01/2024 ZYMFENTRA
infliximab-dyyb injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72606-047 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INFLIXIMAB-DYYB (UNII: B72HH48FLU) (INFLIXIMAB - UNII:B72HH48FLU) INFLIXIMAB-DYYB 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) 0.19 mg in 1 mL SODIUM ACETATE (UNII: 4550K0SC9B) 0.56 mg in 1 mL SORBITOL (UNII: 506T60A25R) 45 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.5 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72606-047-01 1 in 1 CARTON 03/01/2024 1 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761358 03/01/2024 Labeler - CELLTRION USA Inc. (116587378)
Trademark Results [ZYMFENTRA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZYMFENTRA 79378816 not registered Live/Pending |
CELLTRION, INC. 2023-08-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.