VETADRYL 10- diphenhydramine hydrochloride tablet VETADRYL 30- diphenhydramine hydrochloride tablet
Vetadryl 30 by
Drug Labeling and Warnings
Vetadryl 30 by is a Animal medication manufactured, distributed, or labeled by Pegasus Laboratories, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Description:
-
Directions for use:
Diphenhydramine HCl has been used as an aid in the management of allergies, insect bites, motion sickness, travel anxiety, and other conditions. Use as instructed by a licensed veterinarian.
Dosage Information:
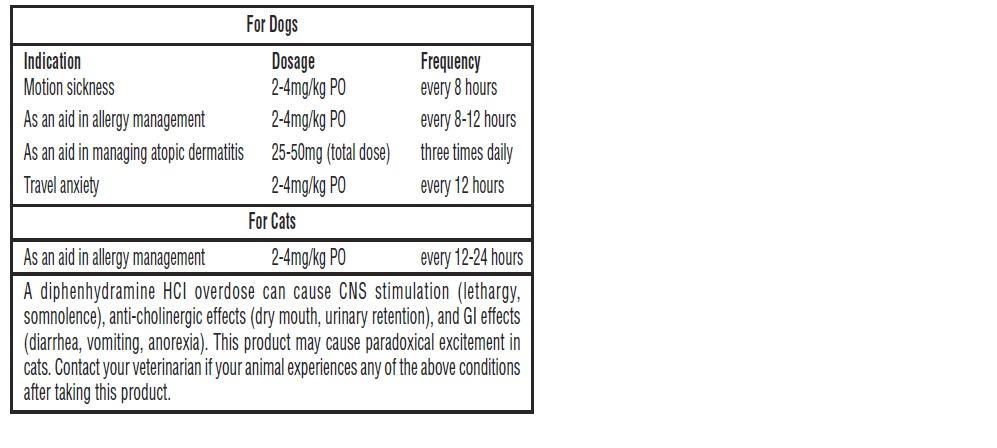
For Dogs Indication Dosage Frequency Motion sickness 2-4mg/kg PO every 8 hours As an aid in allergy management 2-4mg/kg PO every 8-12 hours As an aid in managing atopic dermatitis 25-50mg (total dose) three times daily Travel anxiety 2-4mg/kg PO every 12 hours For Cats Indication Dosage Frequency As an aid in allergy management 2-4mg/kg PO every 12-24 hours - Each scored tablet contains
- Storage:
-
Caution:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
KEEP OUT OF REACH OF CHILDREN AND ANIMALS
FOR VETERINARY USE ONLY
NOT FOR HUMAN USE
The safety of this product in breeding animals is unknown. Do not use this product in breeding animals.
Overdose Information:
A diphenhydramine overdose can cause CNS stimulation (lethargy, somnolence), anti-cholinergic effects (dry mouth, urinary retention), and GI effects (diarrhea, vomiting, anorexia). This product may cause paradoxical excitement in cats. Contact your veterinarian if your animal experiences any of the above conditions after taking this product.
-
Principal Display Panel:
PRN PHARMACAL NDC #49427-329-45 or NDC #49427-330-45 or NDC #49427-329-57 or NDC #49427-330-57
VETADRYL 10/30
A flavored Diphenhydramine HCl
tablet for dogs and cats
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
NET CONTENTS:
30/250 Tablets
Manufactured for
PRN PHARMACAL
Employee-Owned
PENSACOLA, FL. 32514
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VETADRYL 10
diphenhydramine hydrochloride tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 49427-329 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 10 mg Product Characteristics Color brown Score 2 pieces Shape ROUND (Scored on one side imprint code on other side) Size 6mm Flavor LIVER (Dried Chicken Liver Flavor) Imprint Code 10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49427-329-45 12 in 1 CARTON 1 250 in 1 BOTTLE 2 NDC: 49427-329-57 12 in 1 CARTON 2 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/31/2012 VETADRYL 30
diphenhydramine hydrochloride tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 49427-330 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 30 mg Product Characteristics Color brown Score 2 pieces Shape ROUND (Scored on one side imprint code on other side) Size 8mm Flavor LIVER (Dried Chicken Liver Flavor) Imprint Code 30 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49427-330-45 12 in 1 CARTON 1 250 in 1 BOTTLE 2 NDC: 49427-330-57 12 in 1 CARTON 2 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/31/2012 Labeler - Pegasus Laboratories, Inc (108454760) Registrant - Pegasus Laboratories, Inc (108454760) Establishment Name Address ID/FEI Business Operations Pegasus Laboratories, Inc 108454760 manufacture, analysis, label
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.