Q SHIELD- benzalkonium chloride liquid
Q Shield by
Drug Labeling and Warnings
Q Shield by is a Otc medication manufactured, distributed, or labeled by Pierce Innovations, LLC, Fill Tech USA, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
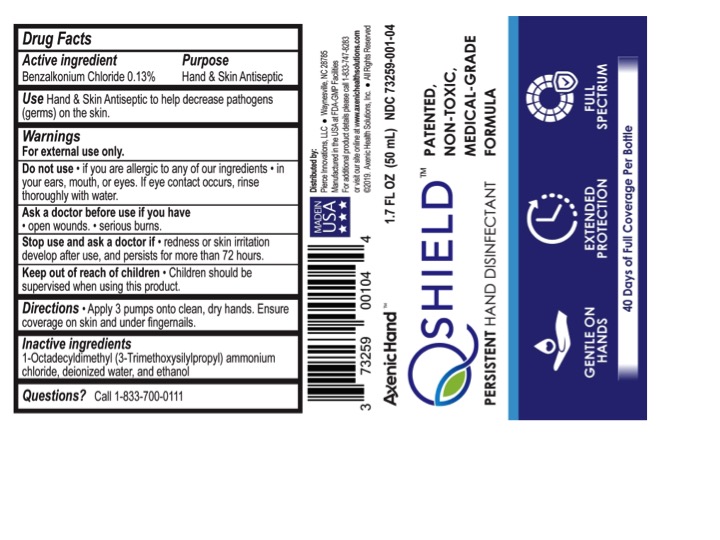
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- Questions?
-
Principal Display Panel
Axenic Hand(tm)
Q Shield(tm)
Patented, Non-Toxic, Medical-grade Formula
Persistent Hand Disinfectant
Gentle on Hands
Extended Protection
Full Spectrum
40 Days of Full Coverage Per Bottle
Made in USA
(Distributed by:) (Manufactured for:)
Pierce Innovations, LLC
4578 Jonathan Creek Road
Waynesville, NC 28785
Manufactured in the USA at FDA-GMP Facilities
For additional product details, please call 1-833-747-8283 or visit our site online at www.axenichealthsolutions.com
(c) 2019. Axenic Health Solutions, Inc. All Rights Reserved
XX fl oz (XX mL) NDC: 73259-001-XX

-
INGREDIENTS AND APPEARANCE
Q SHIELD
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 73259-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength DIMETHYLOCTADECYL(3-(TRIMETHOXYSILYL)PROPYL)AMMONIUM CHLORIDE (UNII: IQ36O85WQ4) ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73259-001-01 550 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 10/15/2019 2 NDC: 73259-001-04 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 10/15/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 10/15/2019 Labeler - Pierce Innovations, LLC (091560426) Establishment Name Address ID/FEI Business Operations Fill Tech USA, LLC 926433855 label(73259-001) , pack(73259-001) , repack(73259-001) , relabel(73259-001) , manufacture(73259-001)
Trademark Results [Q Shield]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 Q SHIELD 98666024 not registered Live/Pending |
Tex Tech Industries, Inc. 2024-07-25 |
 Q SHIELD 97501508 not registered Live/Pending |
ILDONG BIOSCIENCE CO., LTD. 2022-07-13 |
 Q SHIELD 90456582 not registered Live/Pending |
Axenic Health Solutions, Inc. 2021-01-09 |
 Q SHIELD 88951552 not registered Live/Pending |
Q Shield Inc 2020-06-06 |
 Q SHIELD 88951533 not registered Live/Pending |
Q Shield Inc 2020-06-06 |
 Q SHIELD 88235226 5855577 Live/Registered |
Total Quality Maintenance, LLC 2018-12-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.