CVS HEALTH ALL PURPOSE KIT- isopropyl alcohol, bacitracin zinc,neomycin sulfate,polymyxin b sulfate, benzocaine kit
CVS Health All Purpose Kit by
Drug Labeling and Warnings
CVS Health All Purpose Kit by is a Otc medication manufactured, distributed, or labeled by CVS Pharmacy Inc, Tender Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- Stop use and ask a doctor if
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
- Inactive Ingredient
- ACTIVE INGREDIENTS
- PURPOSE
- USE
- WARNINGS
- DO NOT USE
- Ask a doctor before use if you have
- STOP USE AND ASK A DOCTOR IF
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Inactive Ingredients
- ACTIVE INGREDIENTS
- PURPOSE
- USE
- WARNINGS
- DO NOT USE
- STOP USE AND ASK A DOCTOR IF
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENT
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- DO NOT USE
- STOP USE AND ASK A DOCTOR IF
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- INACTIVE INGREDIENTS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVS HEALTH ALL PURPOSE KIT
isopropyl alcohol, bacitracin zinc,neomycin sulfate,polymyxin b sulfate, benzocaine kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69842-419 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69842-419-01 1 in 1 KIT; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 09/01/2019 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 12 POUCH 6 mL Part 2 3 TUBE 2.7 g Part 3 3 PACKAGE 3000 mg Part 4 2 PACKET 1.8 g Part 1 of 4 EASY CARE FIRST AID SKIN CLEANSING ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 44224-0010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44224-0010-0 0.5 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/02/2019 Part 2 of 4 EASY CARE FIRST AID TRIPLE ANTIBIOTIC
bacitracin zinc,neomycin sulfate,polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC: 44224-0013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN ZINC 400 [iU] in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44224-0013-4 0.9 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 09/01/2019 Part 3 of 4 AFTER BITE WIPE INSECT STING RELIEF
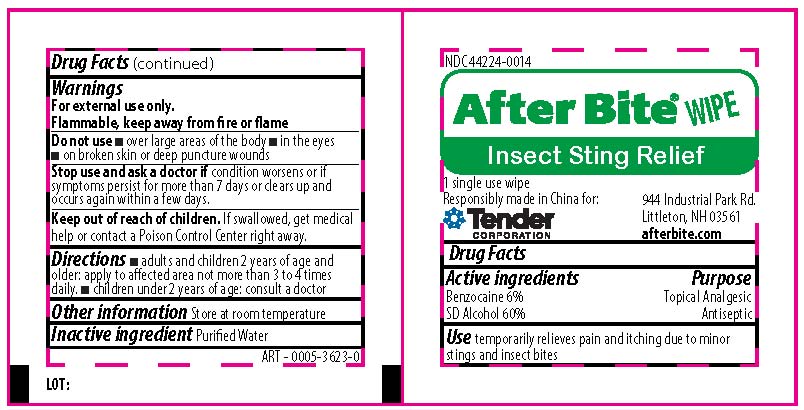
benzocaine,alcohol swabProduct Information Item Code (Source) NDC: 44224-0014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 6 mg in 100 mg ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 60 mg in 100 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44224-0014-1 1000 mg in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 09/01/2019 Part 4 of 4 EASY CARE FIRST AID AFTERBURN
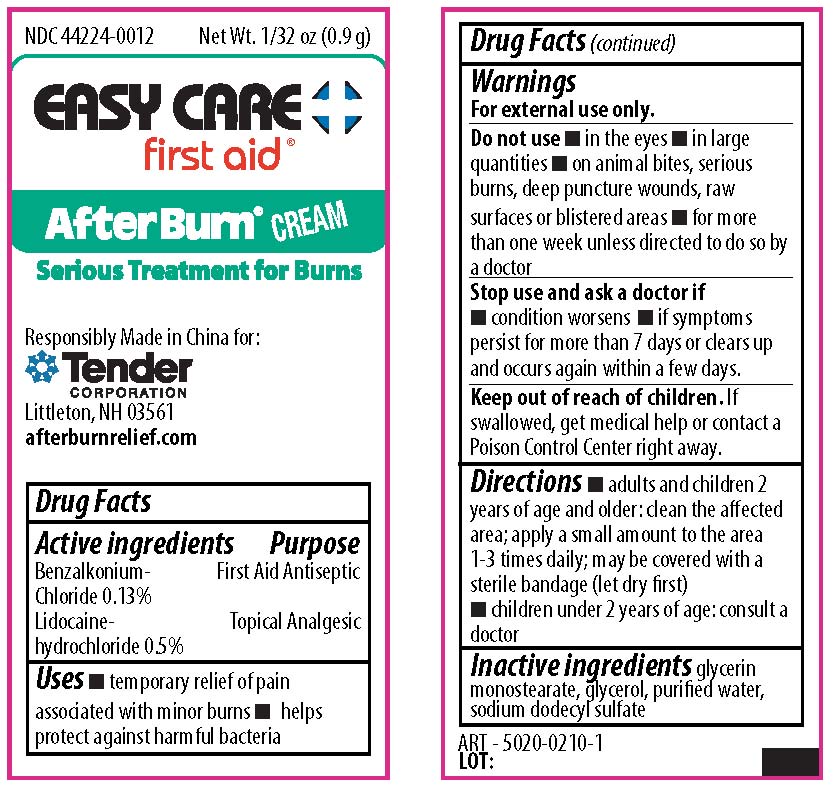
benzalkonium chloride, lidocaine creamProduct Information Item Code (Source) NDC: 44224-0012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 0.5 g in 100 g BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44224-0012-1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/01/2019 Labeler - CVS Pharmacy Inc (062312574) Establishment Name Address ID/FEI Business Operations Tender Corporation 064437304 manufacture(69842-419)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.