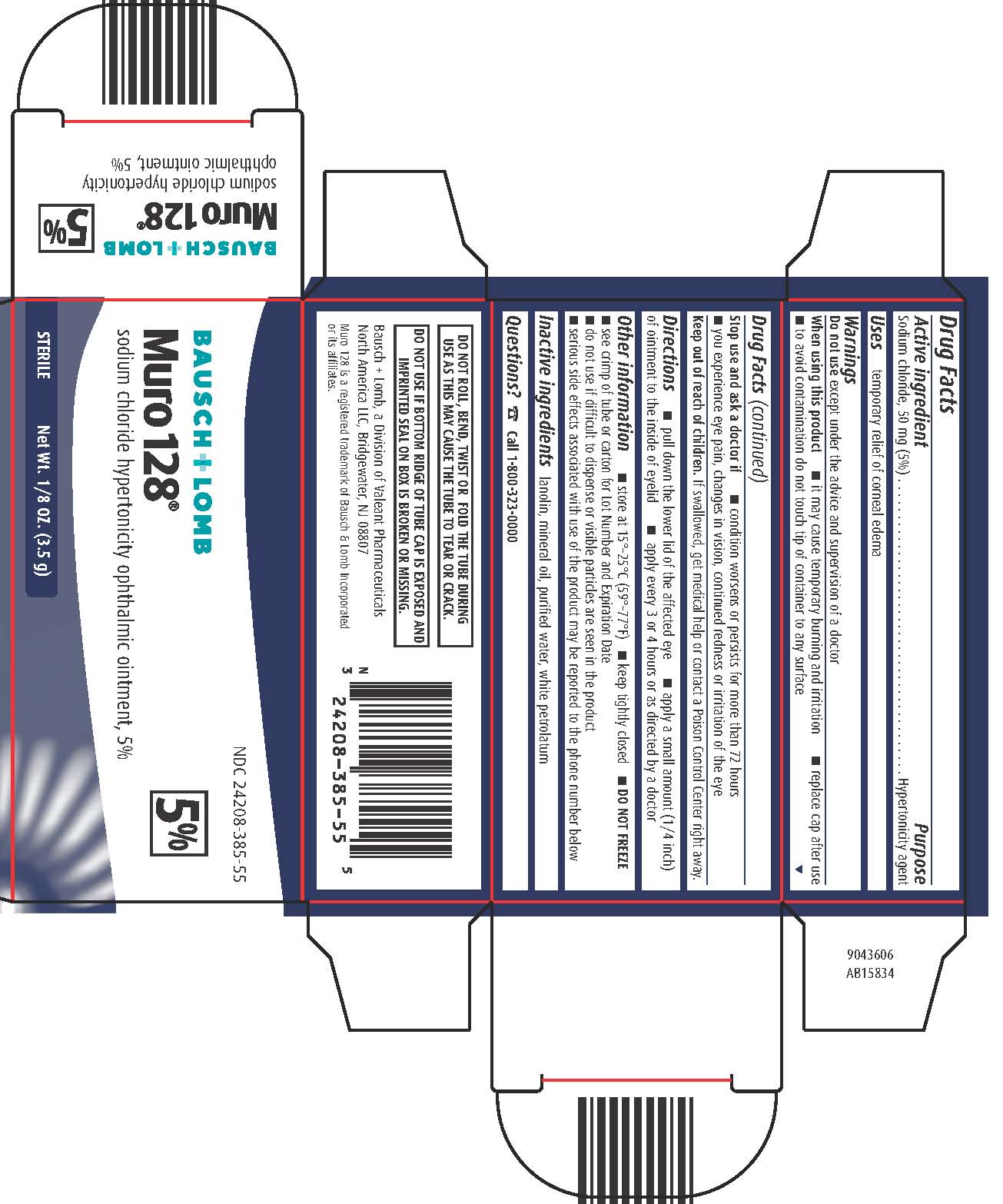
MURO 128- sodium chloride ointment
Muro 128 by
Drug Labeling and Warnings
Muro 128 by is a Otc medication manufactured, distributed, or labeled by Bausch & Lomb Incorporated. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients
- Purpose
- Uses
-
Warnings
Do not use except under the advice and supervision of a doctor
When using this product
- it may cause temporary burning and irritation
- replace cap after use
- to avoid contamination do not touch tip of container to any surface
Stop use and ask a doctor if
- condition worsens or persists for more than 72 hours
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- Keep out of reach of children.
- Directions
-
Other information
- store at 15° - 25°C (59° - 77°F)
- keep tightly closed
- DO NOT FREEZE
- see crimp of tube or carton for Lot Number and Expiration Date
- do not use if difficult to dispense or visible particles are seen in the product
- serious side effects associated with use of the product may be reported to the phone number below
- Inactive ingredients
- Questions?
-
Package/Label Principal Display Panel
NDC: 24208-385-55
Bausch & Lomb
Muro 128®
sodium chloride hypertonicity ophthalmic ointment, 5%
STERILE Net Wt. 1/8 OZ. (3.5 g)
-
INGREDIENTS AND APPEARANCE
MURO 128
sodium chloride ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 24208-385 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength LANOLIN (UNII: 7EV65EAW6H) WATER (UNII: 059QF0KO0R) PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 24208-385-55 1 in 1 CARTON 01/01/2011 1 3.5 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 24208-385-56 2 in 1 CARTON 01/01/2011 2 3.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 01/01/2011 Labeler - Bausch & Lomb Incorporated (196603781) Establishment Name Address ID/FEI Business Operations Bausch & Lomb Incorporated 079587625 MANUFACTURE(24208-385)
Trademark Results [Muro 128]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MURO 128 78905949 3241901 Live/Registered |
BAUSCH & LOMB INCORPORATED 2006-06-12 |
 MURO 128 73278983 1251448 Dead/Cancelled |
Muro Pharmaceutical, Inc. 1980-09-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.