5% DEXTROSE IN WATER FOR INJECTION
Dextrose by
Drug Labeling and Warnings
Dextrose by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation, Baxter, S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DEXTROSE- dextrose monohydrate injection
Baxter Healthcare Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
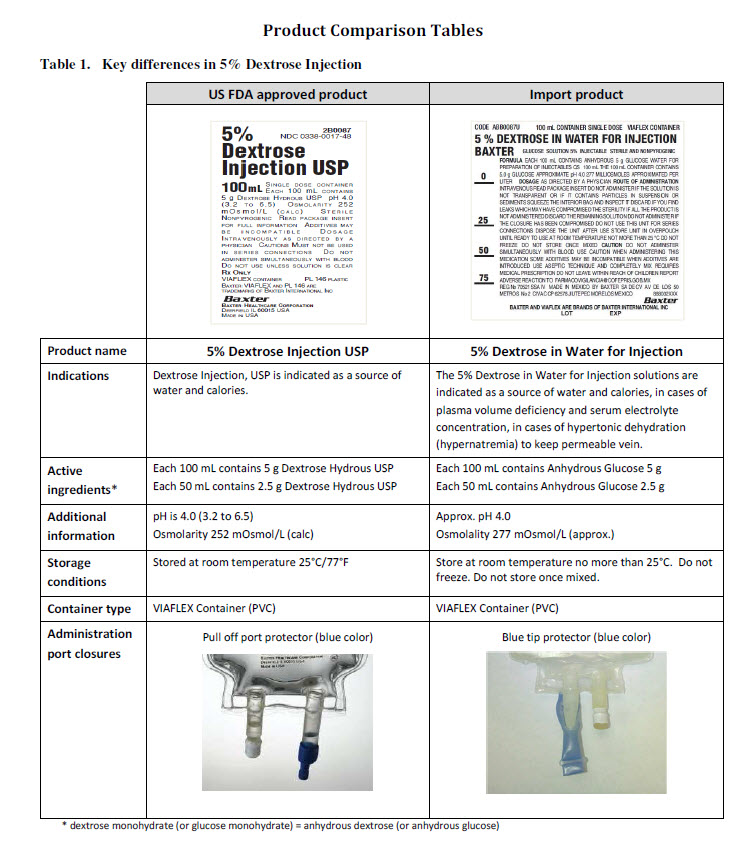
5% DEXTROSE IN WATER FOR INJECTION
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
CODE ABB0086U
50 mL CONTAINER SINGLE DOSE
VIAFLEX CONTAINER
5% DEXTROSE IN WATER FOR INJECTION BAXTER
GLUCOSE SOLUTION 5 % INJECTABLE STERILE AND NONPYROGENIC
FORMULA EACH 100 mL CONTAINS 5 G ANHYDROUS GLUCOSE WATER FOR
PREPARATION OF INJECTABLES QS 100 mL THE 50 mL CONTAINER CONTAINS 2.5 g
GLUCOSE APPROXIMATE pH 4 277 MILLIOSMOLES APPROXIMATED PER LITER DOSAGE
AS DIRECTED BY A PHYSICIAN ROUTE OF ADMINISTRATION INTRAVENOUS READ
PACKAGE INSERT DO NOT ADMINISTER IF THE SOLUTION IS NOT TRANSPARENT OR IF IT
CONTAINS PARTICLES IN SUSPENSION OR SEDIMENTS SQUEEZE THE INTERIOR BAG
AND INSPECT IT DISCARD IF YOU FIND LEAKS WHICH MAY HAVE COMPROMISED THE
STERILITY IF ALL THE PRODUCT IS NOT ADMINISTERED DISCARD THE REMAINING
SOLUTION DO NOT ADMINISTER IF THE CLOSURE HAS BEEN COMPROMISED DO NOT
USE THIS UNIT FOR SERIES CONNECTIONS DISPOSE THE UNIT AFTER USE STORE UNIT
IN OVERPOUCH UNTIL READY TO USE AT ROOM TEMPERATURE NOT MORE THAN 25 ºC
DO NOT FREEZE DO NOT STORE ONCE MIXED CAUTION DO NOT ADMINISTER
SIMULTANEOUSLY WITH BLOOD USE CAUTION WHEN ADMINISTERING THIS
MEDICATION SOME ADDITIVES MAY BE INCOMPATIBLE WHEN ADDITIVES ARE
INTRODUCED USE ASEPTIC TECHNIQUE AND COMPLETELY MIX REQUIRES MEDICAL
PRESCRIPTION DO NOT LEAVE WITHIN REACH OF CHILDREN REPORT ADVERSE
REACTION TO FARMACOVIGILANCIA@COFEPRIS.GOB.MX REG No 70521 SSA IV MADE
IN MEXICO BY BAXTER SA DE CV AV DE LOS 50 METROS No 2 CIVAC CP 62578 JIUTEPEC
MORELOS MEXICO 88 80 32 1001 Baxter Logo
BAXTER AND VIAFLEX ARE BRANDS OF BAXTER INTERNATIONAL INC
LOT
EXP
0
25
CODE ABB0087U
100 mL CONTAINER SINGLE DOSE
VIAFLEX CONTAINER
5% DEXTROSE IN WATER FOR INJECTION BAXTER
GLUCOSE SOLUTION 5 % INJECTABLE STERILE AND NONPYROGENIC
FORMULA EACH 100 mL CONTAINS ANHYDROUS 5 g GLUCOSE WATER FOR
PREPARATION OF INJECTABLES QS 100 mL THE 100 mL CONTAINER CONTAINS
5.0 g GLUCOSE APPROXIMATE pH 4.0 277 MILLIOSMOLES APPROXIMATED PER
LITER DOSAGE AS DIRECTED BY A PHYSICIAN ROUTE OF ADMINISTRATION INTRAVENOUS READ PACKAGE INSERT DO NOT ADMINISTER IF THE SOLUTION IS
NOT TRANSPARENT OR IF IT CONTAINS PARTICLES IN SUSPENSION OR
SEDIMENTS SQUEEZE THE INTERIOR BAG AND INSPECT IT DISCARD IF YOU FIND
LEAKS WHICH MAY HAVE COMPROMISED THE STERILITY IF ALL THE PRODUCT IS
NOT ADMINISTERED DISCARD THE REMAINING SOLUTION DO NOT ADMINISTER IF
THE CLOSURE HAS BEEN COMPROMISED DO NOT USE THIS UNIT FOR SERIES
CONNECTIONS DISPOSE THE UNIT AFTER USE STORE UNIT IN OVERPOUCH
UNTIL READY TO USE AT ROOM TEMPERATURE NOT MORE THAN 25 ºC DO NOT
FREEZE DO NOT STORE ONCE MIXED CAUTION DO NOT ADMINISTER
SIMULTANEOUSLY WITH BLOOD USE CAUTION WHEN ADMINISTERING THIS
MEDICATION SOME ADDITIVES MAY BE INCOMPATIBLE WHEN ADDITIVES ARE
INTRODUCED USE ASEPTIC TECHNIQUE AND COMPLETELY MIX REQUIRES
MEDICAL PRESCRIPTION DO NOT LEAVE WITHIN REACH OF CHILDREN REPORT
ADVERSE REACTION TO FARMACOVIGILANCIA@COFEPRIS.GOB.MX
REG No 70521 SSA IV MADE IN MEXICO BY BAXTER SA DE CV AV DE LOS 50
METROS No 2 CIVAC CP 62578 JIUTEPEC MORELOS MEXICO 88 80 32 1002
Baxter Logo
BAXTER AND VIAFLEX ARE BRANDS OF BAXTER INTERNATIONAL INC
LOT
EXP
0
25
50
75
| DEXTROSE
dextrose monohydrate injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| DEXTROSE
dextrose monohydrate injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter, S.A. de C.V. | 810432484 | ANALYSIS(0338-9521, 0338-9527) , MANUFACTURE(0338-9521, 0338-9527) , LABEL(0338-9521, 0338-9527) , PACK(0338-9521, 0338-9527) , STERILIZE(0338-9521, 0338-9527) | |