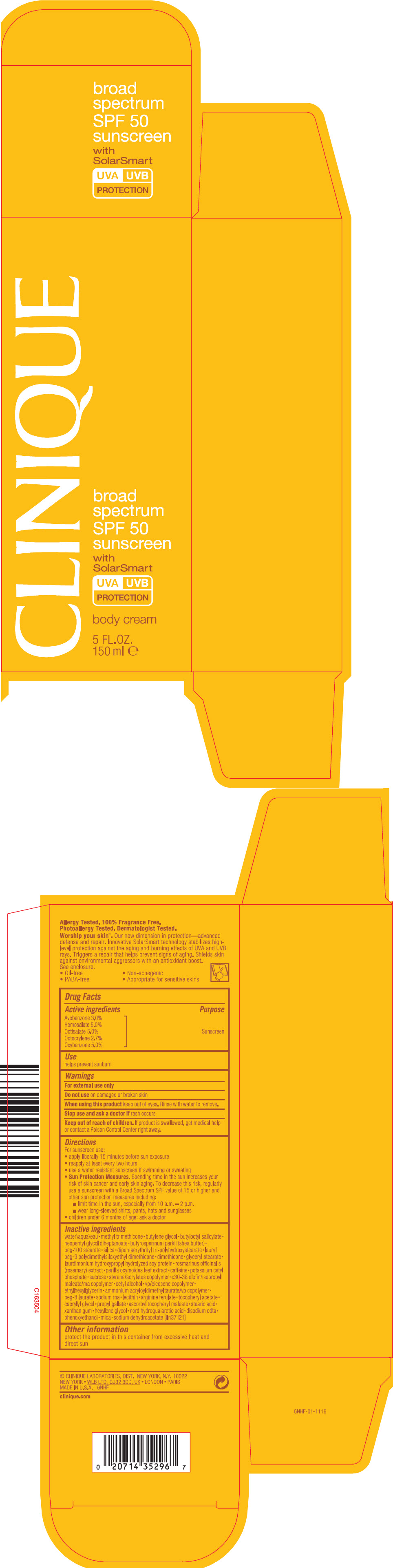
CLINIQUE BROAD SPECTRUM SPF 50 SUNSCREEN BODY WITH SOLAR SMART- avobenzone, homosalate, octisalate, octocrylene, and oxybenzone cream
CLINIQUE BROAD SPECTRUM SPF 50 SUNSCREEN BODY WITH SOLAR SMART by
Drug Labeling and Warnings
CLINIQUE BROAD SPECTRUM SPF 50 SUNSCREEN BODY WITH SOLAR SMART by is a Otc medication manufactured, distributed, or labeled by CLINIQUE LABORATORIES LLC, Estee Lauder Companies Inc., PALC, Estee Lauder Cosmetics Ltd., Whitman Laboratories Ltd., Estee Lauder N.V., The Estee Lauder Inc, Northtec LLC, PADC 1. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
water\aqua\eau methyl trimethicone butylene glycol butyloctyl salicylate neopentyl glycol diheptanoate butyrospermum parkii (shea butter) peg-100 stearate silica dipentaerythrityl tri-polyhydroxystearate lauryl peg-9 polydimethylsiloxyethyl dimethicone dimethicone glyceryl stearate laurdimonium hydroxypropyl hydrolyzed soy protein rosmarinus officinalis (rosemary) extract perilla ocymoides leaf extract caffeine potassium cetyl phosphate sucrose styrene/acrylates copolymer c30-38 olefin/isopropyl maleate/ma copolymer cetyl alcohol vp/eicosene copolymer ethylhexylglycerin ammonium acryloyldimethyltaurate/vp copolymer peg-8 laurate sodium rna lecithin arginine ferulate tocopheryl acetate caprylyl glycol propyl gallate ascorbyl tocopheryl maleate stearic acid xanthan gum hexylene glycol nordihydroguaiaretic acid disodium edta phenoxyethanol mica sodium dehydroacetate [iln37121]
- Other information
- PRINCIPAL DISPLAY PANEL - 150 ml Tube Carton
-
INGREDIENTS AND APPEARANCE
CLINIQUE BROAD SPECTRUM SPF 50 SUNSCREEN BODY WITH SOLAR SMART
avobenzone, homosalate, octisalate, octocrylene, and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49527-069 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.0309 g in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 0.0515 g in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 0.0515 g in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.0278 g in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 0.0515 g in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) methyl trimethicone (UNII: S73ZQI0GXM) butylene glycol (UNII: 3XUS85K0RA) butyloctyl salicylate (UNII: 2EH13UN8D3) neopentyl glycol diheptanoate (UNII: 5LKW3C543X) shea butter (UNII: K49155WL9Y) peg-100 stearate (UNII: YD01N1999R) silicon dioxide (UNII: ETJ7Z6XBU4) dipentaerythrityl tri-polyhydroxystearate (UNII: D21K655H52) lauryl peg-9 polydimethylsiloxyethyl dimethicone (UNII: 25G622K2RA) dimethicone (UNII: 92RU3N3Y1O) glyceryl monostearate (UNII: 230OU9XXE4) rosemary (UNII: IJ67X351P9) caffeine (UNII: 3G6A5W338E) potassium cetyl phosphate (UNII: 03KCY6P7UT) sucrose (UNII: C151H8M554) cetyl alcohol (UNII: 936JST6JCN) ethylhexylglycerin (UNII: 147D247K3P) ammonium acryloyldimethyltaurate/vp copolymer (UNII: W59H9296ZG) peg-8 laurate (UNII: 762O8IWA10) arginine ferulate (UNII: 0774Y45BEE) caprylyl glycol (UNII: 00YIU5438U) propyl gallate (UNII: 8D4SNN7V92) ascorbyl tocopheryl maleate (UNII: D2G6259XR5) stearic acid (UNII: 4ELV7Z65AP) xanthan gum (UNII: TTV12P4NEE) hexylene glycol (UNII: KEH0A3F75J) edetate disodium anhydrous (UNII: 8NLQ36F6MM) phenoxyethanol (UNII: HIE492ZZ3T) mica (UNII: V8A1AW0880) sodium dehydroacetate (UNII: 8W46YN971G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49527-069-01 1 in 1 CARTON 06/01/2011 1 150 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 06/01/2011 Labeler - CLINIQUE LABORATORIES INC. (173047747) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COMPANY, THE 828534516 RELABEL(49527-069) , REPACK(49527-069) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 244669714 MANUFACTURE(49527-069) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD. 202952982 MANUFACTURE(49527-069) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 MANUFACTURE(49527-069) Establishment Name Address ID/FEI Business Operations LEN-RON MANUFACTURING DIVISION OF ARAMIS INC 809771152 MANUFACTURE(49527-069) Establishment Name Address ID/FEI Business Operations NORTHTEC INC 943871157 RELABEL(49527-069) , REPACK(49527-069) Establishment Name Address ID/FEI Business Operations PADC 1 949264774 RELABEL(49527-069) , REPACK(49527-069) Establishment Name Address ID/FEI Business Operations WHITMAN LABORATORIES, LTD. 216866277 MANUFACTURE(49527-069) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 255175580 MANUFACTURE(49527-069)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.