INSADOL (corn sterilized quantitative extract- beta sitosterol tablet
INSADOL by
Drug Labeling and Warnings
INSADOL by is a Otc medication manufactured, distributed, or labeled by LYDIA Co., Ltd, I World Pharmaceutical Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
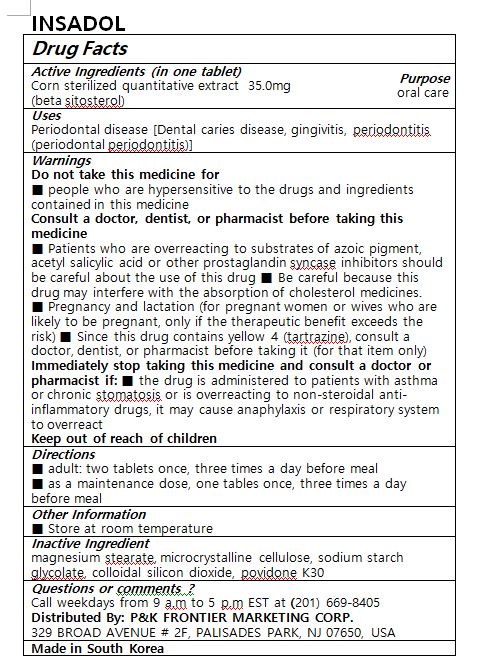
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Do not take this medicine if
■ people who are hypersensitive to the drugs and ingredients contained in this medicine
Consult a doctor, dentist, or pharmacist before taking this medicine
■ Patients who are overreacting to substrates of azoic pigment, acetyl salicylic acid or other prostaglandin syncase inhibitors should be careful about the use of this drug ■ Be careful because this drug may interfere with the absorption of cholesterol medicines.
■ Pregnancy and lactation (for pregnant women or wives who are likely to be pregnant, only if the therapeutic benefit exceeds the risk) ■ Since this drug contains yellow 4 (tartrazine), consult a doctor, dentist, or pharmacist before taking it (for that item only)
Immediately stop taking this medicine and consult a doctor or pharmacist if: ■ the drug is administered to patients with asthma or chronic stomatosis or is overreacting to non-steroidal anti-inflammatory drugs, it may cause anaphylaxis or respiratory system to overreact
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INSADOL
corn sterilized quantitative extract (beta sitosterol) tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72988-0016 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .BETA.-SITOSTEROL (UNII: S347WMO6M4) (.BETA.-SITOSTEROL - UNII:S347WMO6M4) .BETA.-SITOSTEROL 35 mg Inactive Ingredients Ingredient Name Strength POVIDONE K30 (UNII: U725QWY32X) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) POWDERED CELLULOSE (UNII: SMD1X3XO9M) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color orange Score no score Shape ROUND Size 10mm Flavor Imprint Code ISD Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72988-0016-1 100 in 1 BLISTER PACK; Type 0: Not a Combination Product 10/08/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/08/2019 Labeler - LYDIA Co., Ltd (695735569) Registrant - LYDIA Co., Ltd (695735569) Establishment Name Address ID/FEI Business Operations I World Pharmaceutical Co., Ltd 688222857 manufacture(72988-0016) Establishment Name Address ID/FEI Business Operations LYDIA Co., Ltd 695735569 label(72988-0016)
Trademark Results [INSADOL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 INSADOL 97575733 not registered Live/Pending |
Kim, Anthony H. 2022-09-01 |
 INSADOL 72229032 0813338 Dead/Expired |
SERDEX-SOCIETE D'ETUDES DE RECHERCHES DE DIFFUSION ET D'EXPLOITATION 1965-09-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.