MADECASSOL- neomycin sulfate, titrated ext. of centella asiatica ointment
MADECASSOL by
Drug Labeling and Warnings
MADECASSOL by is a Otc medication manufactured, distributed, or labeled by LYDIA Co., Ltd, I World Pharmaceutical Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
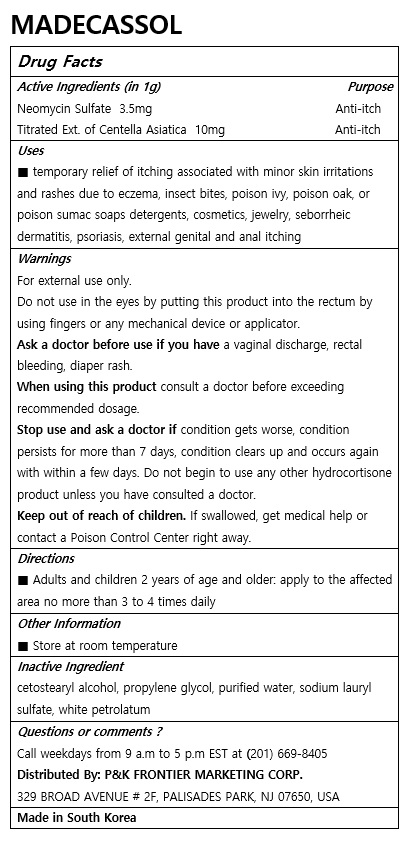
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
For external use only.
Do not use in the eyes by putting this product into the rectum by using fingers or any mechanical device or applicator.
Ask a doctor before use if you have a vaginal discharge, rectal bleeding, diaper rash.
When using this product consult a doctor before exceeding recommended dosage.
Stop use and ask a doctor if condition gets worse, condition persists for more than 7 days, condition clears up and occurs again with within a few days. Do not begin to use any other hydrocortisone product unless you have consulted a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MADECASSOL
neomycin sulfate, titrated ext. of centella asiatica ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72988-0018 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CENTELLA ASIATICA (UNII: 7M867G6T1U) (CENTELLA ASIATICA - UNII:7M867G6T1U) CENTELLA ASIATICA 10 mg in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 3.5 mg in 1 g Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72988-0018-1 10 g in 1 TUBE; Type 0: Not a Combination Product 10/08/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/08/2019 Labeler - LYDIA Co., Ltd (695735569) Registrant - LYDIA Co., Ltd (695735569) Establishment Name Address ID/FEI Business Operations I World Pharmaceutical Co., Ltd 688222857 manufacture(72988-0018) Establishment Name Address ID/FEI Business Operations LYDIA Co., Ltd 695735569 label(72988-0018)
Trademark Results [MADECASSOL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MADECASSOL 73301973 1200816 Dead/Cancelled |
Laroche Navarron S.A. 1981-03-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.