VOSTALLY- ramipril solution
Vostally by
Drug Labeling and Warnings
Vostally by is a Prescription medication manufactured, distributed, or labeled by Metacel Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VOSTALLY® safely and effectively. See full prescribing information for VOSTALLY.
VOSTALLY (ramipril) oral solution
Initial U.S. Approval: 1991INDICATIONS AND USAGE
VOSTALLY is an angiotensin converting enzyme (ACE) inhibitor indicated:
- for the treatment of hypertension in adults, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions ( 1.1).
- In patients 55 years or older at high risk of developing a major cardiovascular event, VOSTALLY is indicated to reduce the risk of myocardial infarction, stroke, or death from cardiovascular causes ( 1.2).
- In adult patients with post-myocardial infarction heart failure to reduce the risk of cardiovascular death and hospitalization for heart failure ( 1.3).
DOSAGE AND ADMINISTRATION
- Hypertension: Initial dose is 2.5 mg to 20 mg orally once daily. Adjust dosage according to blood pressure response after 2–4 weeks of treatment. The usual maintenance dose following titration is 2.5 mg to 20 mg orally daily as a single dose or equally divided doses ( 2.2).
- Reduction in the risk of myocardial infarction, stroke, or death from cardiovascular causes: 2.5 mg orally once daily for 1 week, 5 mg orally once daily for 3 weeks, and increased as tolerated to a maintenance dose of 10 mg orally once daily ( 2.3).
- Heart failure post-myocardial infarction: Starting dose of 2.5 mg orally twice daily. If patient becomes hypotensive at this dose, decrease dosage to 1.25 mg orally twice daily. Titrate at 3-week intervals as tolerated to a target dose of 5 mg orally twice daily ( 2.4).
CONTRAINDICATIONS
- Angioedema or hypersensitivity related to previous treatment with an ACE inhibitor, or a history of hereditary or idiopathic angioedema ( 4).
- VOSTALLY is contraindicated in combination with a neprilysin inhibitor (e.g., sacubitril). Do not administer VOSTALLY within 36 hours of switching to or from sacubitril/valsartan, a neprilysin inhibitor ( 4).
- Do not co-administer aliskiren with VOSTALLY in patients with diabetes ( 4).
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions included cough and hypotension ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Rosemont Pharmaceuticals, LLC at 1-844-638-2235 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- Diuretics: Excessive drop in blood pressure ( 7.1).
- Potassium-sparing diuretics/potassium supplements: Hyperkalemia ( 7.2)
- Dual inhibition of the renin-angiotensin system: Increased risk of renal impairment, hypotension, and hyperkalemia ( 7.3)
- Lithium: Increase serum lithium levels, symptoms of lithium toxicity ( 7.4).
- Gold: Nitritoid reactions have been reported ( 7.5).
- NSAID use may lead to increased risk of renal impairment and loss of antihypertensive effect ( 7.6).
- mTOR inhibitor or neprilysin inhibitor use may increase angioedema risk ( 7.7).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: FETAL TOXICITY
1 INDICATIONS AND USAGE
1.1 Hypertension
1.2 Reduction in the Risk of Myocardial Infarction, Stroke, and Death from Cardiovascular Causes
1.3 Post-Myocardial Infarction Heart Failure
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
2.2 Recommended Dosage in Hypertension
2.3 Recommended Dosage in Reduction in Risk of Myocardial Infarction, Stroke, and Death from Cardiovascular Causes
2.4 Recommended Dosage in Post-Myocardial Infarction Heart Failure
2.5 Dosage Adjustments
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
5.2 Angioedema and Anaphylactoid Reactions
5.3 Hypotension
5.4 Hepatic Failure
5.5 Worsening Renal Function
5.6 Hyperkalemia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Diuretics
7.2 Agents Increasing Serum Potassium
7.3 Dual Blockade of the Renin-Angiotensin-Aldosterone System
7.4 Lithium
7.5 Gold
7.6 Non-Steroidal Anti-Inflammatory Agents including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
7.7 mTOR Inhibitors or Other Drugs Known to Cause Angioedema
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Impaired Liver Function
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Hypertension
14.2 Reduction in Risk of Myocardial Infarction, Stroke, and Death from Cardiovascular Causes
14.3 Post-Myocardial Infarction Heart Failure
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue VOSTALLY as soon as possible [see Warnings and Precautions (5.1)] .
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions (5.1)] .
-
1 INDICATIONS AND USAGE
1.1 Hypertension
VOSTALLY is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including this drug.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
VOSTALLY may be used alone or in combination with thiazide diuretics.
1.2 Reduction in the Risk of Myocardial Infarction, Stroke, and Death from Cardiovascular Causes
VOSTALLY is indicated in patients 55 years or older at high risk of developing a major cardiovascular event because of a history of coronary artery disease, stroke, peripheral vascular disease, or diabetes that is accompanied by at least one other cardiovascular risk factor (hypertension, elevated total cholesterol levels, low HDL levels, cigarette smoking, or documented microalbuminuria), to reduce the risk of myocardial infarction, stroke, or death from cardiovascular causes.
1.3 Post-Myocardial Infarction Heart Failure
VOSTALLY is indicated in adult patients with post-myocardial infarction heart failure to reduce the risk of cardiovascular death and hospitalization for heart failure [see Clinical Studies (14.3)] .
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
Instruct patients or caregivers to use an oral dosing syringe or oral dosing cup to correctly measure the prescribed amount of medication. Inform patients that oral dosing syringes may be obtained from their pharmacy.
2.2 Recommended Dosage in Hypertension
The recommended initial dose for patients not receiving a diuretic is 2.5 mg orally once a day. Adjust dose according to blood pressure response. The usual maintenance dosage range is 2.5 mg to 20 mg orally per day administered as a single dose or in two equally divided doses. In some patients treated once daily, the antihypertensive effect may diminish toward the end of the dosing interval. In such patients, consider an increase in dosage or twice daily administration.
2.3 Recommended Dosage in Reduction in Risk of Myocardial Infarction, Stroke, and Death from Cardiovascular Causes
Initiate dosing at 2.5 mg orally once daily for 1 week, 5 mg orally once daily for the next 3 weeks, and then increase as tolerated, to a maintenance dose of 10 mg orally once daily. If the patient is hypertensive or recently post-myocardial infarction, VOSTALLY can also be given as a divided dose.
2.4 Recommended Dosage in Post-Myocardial Infarction Heart Failure
For the treatment of patients with post-myocardial infarction heart failure, the recommended starting dose of VOSTALLY is 2.5 mg orally twice daily (5 mg orally per day). A patient who becomes hypotensive at this dose may be switched to 1.25 mg orally twice daily. After one week at the starting dose, increase dose (if tolerated) toward a target dose of 5 mg orally twice daily, with dosage increases being about 3 weeks apart.
After the initial dose of VOSTALLY, observe the patient under medical supervision for at least two hours and until blood pressure has stabilized for at least an additional hour. If possible, reduce the dose of any concomitant diuretic as this may diminish the likelihood of hypotension. The appearance of hypotension after the initial dose of VOSTALLY does not preclude subsequent careful dose titration with the drug, following effective management of the hypotension [see Warnings and Precautions (5.5), Drug Interactions (7.1)] .
2.5 Dosage Adjustments
Renal Impairment
Establish baseline renal function in patients initiating VOSTALLY. In patients with creatinine clearance ≤40 mL/min, 25% of the usual dose of ramipril is expected to produce full therapeutic levels of ramiprilat [see Use in Specific Populations (8.6)] .
Hypertension
For patients with hypertension and renal impairment, the recommended initial dosage of VOSTALLY is 1.25 mg orally once daily. Dosage may be titrated upward until blood pressure is controlled or to a maximum total daily dose of 5 mg orally.
Heart Failure Post-Myocardial Infarction
For patients with heart failure and renal impairment, the recommended initial dosage of VOSTALLY is 1.25 mg orally once daily. The dose may be increased to 1.25 mg orally twice daily, and up to a maximum dose of 2.5 mg orally twice daily depending on clinical response and tolerability.
Volume Depletion or Renal Artery Stenosis
Blood pressure decreases associated with any dose of VOSTALLY depend, in part, on the presence or absence of volume depletion (e.g., past and current diuretic use) or the presence or absence of renal artery stenosis. If such circumstances are suspected to be present, initiate dosing at 1.25 mg orally once daily. Adjust dosage according to blood pressure response.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
VOSTALLY is contraindicated in patients with:
- a history of angioedema or hypersensitivity to this product or any other ACE inhibitor [see Warnings and Precautions (5.2)]
- hereditary or idiopathic angioedema [see Warnings and Precautions (5.2)]
VOSTALLY is contraindicated in combination with a neprilysin inhibitor (e.g., sacubitril). Do not administer VOSTALLY within 36 hours of switching to or from sacubitril/valsartan, a neprilysin inhibitor [see Drug Interactions (7.3)]
Do not co-administer VOSTALLY with aliskiren in patients with diabetes [see Drug Interactions (7.3)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
VOSTALLY can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue VOSTALLY as soon as possible [see Use in Specific Populations (8.1)].
5.2 Angioedema and Anaphylactoid Reactions
Angioedema
Head and Neck Angioedema
Angioedema of the face, extremities, lips, tongue, glottis, and/or larynx, including airway obstruction and some fatal reactions, have occurred in patients treated with ACE inhibitors at any time during treatment. VOSTALLY should be promptly discontinued, and appropriate therapy and monitoring should be provided until complete and sustained resolution of signs and symptoms of angioedema has occurred.
Patients with a history of angioedema unrelated to ACE inhibitor therapy may be at increased risk of angioedema while receiving an ACE inhibitor [see Contraindications (4)]. ACE inhibitors have been associated with a higher rate of angioedema in Black than in non-Black patients. Patients taking concomitant mammalian target of rapamycin (mTOR) inhibitor (e.g., temsirolimus, sirolimus, everolimus) therapy or a neprilysin inhibitor may be at increased risk for angioedema. [see Drug Interactions (7.7)]
Intestinal Angioedema
Intestinal angioedema has been reported in patients treated with ACE inhibitors.
These patients presented with abdominal pain (with or without nausea or vomiting); in some cases, there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Include intestinal angioedema in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
Anaphylactoid Reactions
Anaphylactoid Reactions During Desensitization
Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life- threatening anaphylactoid reactions. In the same patients, these reactions were avoided when ACE inhibitors were temporarily withheld, but they reappeared upon inadvertent rechallenge.
Anaphylactoid Reactions During Dialysis
Anaphylactoid reactions have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. In such patients, dialysis must be stopped immediately, and aggressive therapy for anaphylactoid reactions must be initiated. Symptoms have not been relieved by antihistamines in these situations. In these patients, consideration should be given to using a different type of dialysis membrane or a different class of antihypertensive agent. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
5.3 Hypotension
General Considerations
VOSTALLY can cause symptomatic hypotension, after the initial dose or when the dosage has been increased. Patients at risk of symptomatic hypotension include those with volume and/or salt depletion of any etiology (e.g., high dose diuretic therapy, dietary salt restriction, renal dialysis, diarrhea, or vomiting). Correct volume and/or salt depletion before initiating therapy with VOSTALLY and monitor for the first two weeks of treatment and whenever the dose of VOSTALLY and/or diuretic is increased.
If excessive hypotension occurs, place the patient in a supine position and, if necessary, treat with intravenous infusion of physiological saline. VOSTALLY treatment usually can be continued following restoration of blood pressure and volume.
Heart Failure Post-Myocardial Infarction
In patients with heart failure post-myocardial infarction who are currently being treated with a diuretic, symptomatic hypotension occasionally can occur following the initial dose of VOSTALLY. If the initial dose of 2.5 mg VOSTALLY cannot be tolerated, use an initial dose of 1.25 mg VOSTALLY to avoid excessive hypotension. Consider reducing the dose of concomitant diuretic to decrease the incidence of hypotension.
Congestive Heart Failure
In patients with congestive heart failure, with or without associated renal insufficiency, VOSTALLY may cause excessive hypotension, which may be associated with oliguria or azotemia and rarely, with acute renal failure and death. In such patients, initiate VOSTALLY therapy under close medical supervision and monitor for the first 2 weeks of treatment and whenever the dose of VOSTALLY or diuretic is increased.
Surgery and Anesthesia
In patients undergoing surgery or during anesthesia with agents that produce hypotension, VOSTALLY may block angiotensin II secondary to compensatory renin release. Hypotension that occurs as a result of this mechanism can be corrected by volume expansion.
5.4 Hepatic Failure
ACE inhibitors, including VOSTALLY, have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis and sometimes death. The mechanism of this syndrome is not understood. Discontinue VOSTALLY if patient develops jaundice or marked elevations of hepatic enzymes.
5.5 Worsening Renal Function
Worsening renal function can be caused by ACE inhibitors, including VOSTALLY, as a consequence of inhibiting the renin-angiotensin-aldosterone system in susceptible individuals. In patients whose renal function may depend on the activity of the renin-angiotensin-aldosterone system, (e.g., patients with renal artery stenosis, chronic kidney disease, severe congestive heart failure, or volume depletion), treatment with ACE inhibitors, including VOSTALLY, may be associated with oliguria or progressive azotemia and rarely with acute renal failure or death. In such patients, monitor renal function during the first few weeks of therapy. Consider dose reduction, withholding, or discontinuing VOSTALLY in patients who develop a clinically significant decrease in renal function.
5.6 Hyperkalemia
Drugs that inhibit the renin angiotensin system, including VOSTALLY, can cause hyperkalemia. In clinical trials with ramipril, hyperkalemia (serum potassium >5.7 mEq/L) occurred in approximately 1% of hypertensive patients receiving ramipril. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of other drugs that raise serum potassium levels. Monitor serum potassium periodically in patients treated with VOSTALLY [see Drug Interactions (7.2)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Hypertension
Ramipril has been evaluated for safety in over 4000 patients with hypertension; of these, 1230 patients were studied in U.S. controlled trials, and 1107 were studied in foreign controlled trials. Almost 700 of these patients were treated for at least one year. The overall incidence of reported adverse events was similar in ramipril and placebo patients.
The most common reasons for discontinuation of ramipril were cough (1.0%), dizziness (0.5%), and impotence (0.4%). In a later 1-year study, increased cough was seen in almost 12% of ramipril patients, with about 4% of patients requiring discontinuation of treatment.
Reduction in the Risk of Myocardial Infarction, Stroke, and Death from Cardiovascular Causes
HOPE Study
Safety data in the Heart Outcomes Prevention Evaluation (HOPE) study were collected as reasons for discontinuation or temporary interruption of treatment. The incidence of cough was similar to that seen in the Acute Infarction Ramipril Efficacy (AIRE) trial. The rate of angioedema was the same as in previous clinical trials [see Warnings and Precautions (5.2)] .
Clinical Laboratory Test Findings
Creatinine and Blood Urea Nitrogen: Increases in creatinine levels occurred in 1.2% of patients receiving ramipril alone, and in 1.5% of patients receiving ramipril and a diuretic. Increases in blood urea nitrogen levels occurred in 0.5% of patients receiving ramipril alone and in 3% of patients receiving ramipril with a diuretic. None of these increases required discontinuation of treatment. Increases in these laboratory values are more likely to occur in patients with renal insufficiency or those pretreated with a diuretic and based on experience with other ACE inhibitors, would be expected to be especially likely in patients with renal artery stenosis [see Warnings and Precautions (5.5)] .
Hemoglobin and Hematocrit: Decreases in hemoglobin or hematocrit (a low value and a decrease of 5 g/dL or 5%, respectively) were rare, occurring in 0.4% of patients receiving ramipril alone and in 1.5% of patients receiving ramipril plus a diuretic. No US patients discontinued treatment because of decreases in hemoglobin or hematocrit.
6.2 Post-Marketing Experience
Other Adverse Reactions
Other adverse reactions reported in controlled clinical trials (in less than 1% of ramipril patients,) or rarer events seen in post-marketing experience, include the following (in some, a causal relationship to drug is uncertain):
Cardiovascular: palpitations
General disorders: edema, malaise, weight gain
Hematologic: bone marrow depression, agranulocytosis, neutropenia, pancytopenia, hemolytic anemia, and thrombocytopenia
Gastrointestinal: pancreatitis, abdominal pain, anorexia, constipation, dry mouth, dyspepsia, dysphagia, increased salivation, and taste disturbance
Immune system: eosinophilic pneumonitis, hypersensitivity reactions (manifested by urticaria, pruritus, or rash, with or without fever), toxic epidermal necrolysis, Stevens-Johnson syndrome, an immune symptom complex which may include a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia, fever, vasculitis, eosinophilia, photosensitivity, rash and other dermatologic manifestations
Musculoskeletal and connective tissue: arthralgia, arthritis, myalgia
Neurologic and Psychiatric: anxiety, amnesia, convulsions, depression, hearing loss, insomnia, nervousness, neuralgia, neuropathy, paresthesia, somnolence, tinnitus, tremor, vertigo, and vision disturbances
Respiratory, thoracic and mediastinal: dyspnea, epistaxis
Reproductive system: impotence
Skin and subcutaneous tissue: erythema multiforme, hyperhidrosis, photosensitivity, purpura, onycholysis, pemphigus, pemphigoid
In addition to adverse reactions reported from clinical trials, there have been rare reports of hypoglycemia reported during ramipril therapy when given to patients concomitantly taking oral hypoglycemic agents or insulin. The causal relationship is unknown.
-
7 DRUG INTERACTIONS
7.1 Diuretics
Patients on diuretics, especially those in whom diuretic therapy was recently instituted, may occasionally experience an excessive reduction of blood pressure after initiation of therapy with VOSTALLY. The possibility of hypotensive effects with VOSTALLY can be minimized by either decreasing or discontinuing the diuretic or increasing the salt intake prior to initiation of treatment with VOSTALLY. If this is not possible, reduce the starting dose [see Dosage and Administration (2)].
7.2 Agents Increasing Serum Potassium
Coadministration of VOSTALLY with other drugs that raise serum potassium levels may result in hyperkalemia. Monitor serum potassium in such patients.
7.3 Dual Blockade of the Renin-Angiotensin-Aldosterone System
Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on VOSTALLY and other agents that affect the RAS.
Telmisartan
The ONTARGET trial enrolled 25,620 patients >55 years old with atherosclerotic disease or diabetes with end-organ damage, randomized them to telmisartan only, ramipril only, or the combination, and followed them for a median of 56 months. Patients receiving the combination of telmisartan and ramipril did not obtain any benefit in the composite endpoint of cardiovascular death, MI, stroke and heart failure hospitalization compared to monotherapy, but experienced an increased incidence of clinically important renal dysfunction (death, doubling of serum creatinine, or dialysis) compared with groups receiving telmisartan alone or ramipril alone. Concomitant use of telmisartan and ramipril (including VOSTALLY) is not recommended.
Aliskiren
Do not co-administer aliskiren with VOSTALLY in patients with diabetes. Avoid concomitant use of aliskiren with VOSTALLY in patients with renal impairment (GFR <60 mL/min/1.73 m 2).
7.4 Lithium
Increased serum lithium levels and symptoms of lithium toxicity have been reported in patients receiving ACE inhibitors during therapy with lithium; therefore, frequent monitoring of serum lithium levels is recommended. If a diuretic is also used, the risk of lithium toxicity may be increased.
7.5 Gold
Nitritoid reactions (symptoms include facial flushing, nausea, vomiting and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy including VOSTALLY.
7.6 Non-Steroidal Anti-Inflammatory Agents including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including ramipril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving ramipril and NSAID therapy.
The antihypertensive effect of ACE inhibitors, including ramipril, may be attenuated by NSAIDs.
7.7 mTOR Inhibitors or Other Drugs Known to Cause Angioedema
Patients taking concomitant mTOR inhibitor (e.g. temsirolimus) therapy or a neprilysin inhibitor may be at increased risk for angioedema. [see Warnings and Precautions (5.2)]
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
VOSTALLY can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. When pregnancy is detected, discontinue VOSTALLY as soon as possible.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the general U.S. population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20% respectively.
Clinical Considerations
Disease-associatedMaternal and/or Embryo/Fetal Risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section, and post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death. Pregnant women with hypertension should be carefully monitored and managed accordingly.
Fetal/Neonatal Adverse Reactions
Oligohydramnios in pregnant women who use drugs affecting the renin-angiotensin system in the second and third trimesters of pregnancy can result in the following: reduced fetal renal function leading to anuria and renal failure, fetal lung hypoplasia and skeletal deformations, including skull hypoplasia, hypotension, and death. In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus.
Perform serial ultrasound examinations to assess the intra-amniotic environment. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to VOSTALLY for hypotension, oliguria, and hyperkalemia. If oliguria or hypotension occur in neonates with a history of in utero exposure to VOSTALLY, support blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and substituting for disordered renal function.
8.2 Lactation
Risk Summary
Ingestion of a single 10 mg oral dose of ramipril resulted in undetectable amounts of ramipril and its metabolites in breast milk. However, multiple doses may produce low milk concentrations that are not predictable from a single dose. There are no available data on the effects of ramipril and its metabolites on the breastfed infant or on milk production. Because of the potential for severe adverse reactions in the breastfed infant, including hypotension, hyperkalemia and renal impairment, advise women not to breastfeed during treatment with VOSTALLY.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Irreversible kidney damage has been observed in very young rats given a single dose of ramipril. Use of VOSTALLY is not recommended in children less than 2 years of age. It is not known whether post-natal use of ramipril, before maturation of renal function is complete, has a long-term deleterious effect on the kidney.
Neonates with a history of in utero exposure to ramipril:
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
8.5 Geriatric Use
Of the total number of patients who received ramipril in U.S. clinical studies of ramipril, 11.0% were ≥65 years of age while 0.2% were ≥75 years of age. No overall differences in effectiveness or safety were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but a greater sensitivity of some older individuals cannot be ruled out.
One pharmacokinetic study conducted in hospitalized elderly patients indicated that peak ramiprilat levels and area under the plasma concentration-time curve (AUC) for ramiprilat are higher in older patients.
8.6 Renal Impairment
In patients of creatinine clearance 40-80 mL/min, 15-40 mL/min, and <15 mL/min, the exposure of ramiprilat was approximately 1.7-fold higher, 3.0-fold higher, and 3.2-fold higher respectively, compared to the group with Creatinine clearance >80mL/min. Overall, the results suggest that the starting dose of ramipril should be adjusted in patients with creatinine clearance <40 mL/min [see Dosage and Administration (2.5)].
8.7 Impaired Liver Function
As ramipril is primarily metabolized by hepatic esterases to its active moiety, ramiprilat, patients with impaired liver function could develop markedly elevated plasma levels of ramipril. No formal pharmacokinetic studies have been carried out in hypertensive patients with impaired liver function.
-
10 OVERDOSAGE
Single oral doses of ramipril in rats and mice of 10 g/kg–11 g/kg resulted in significant lethality. In dogs, oral doses as high as 1 g/kg induced only mild gastrointestinal distress. Limited data on human overdosage are available. The most likely clinical manifestations would be symptoms attributable to hypotension.
Laboratory determinations of serum levels of ramipril and its metabolites are not widely available, and such determinations have, in any event, no established role in the management of ramipril overdose.
No data are available to suggest physiological maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of ramipril and its metabolites. Similarly, it is not known which, if any, of these substances can be effectively removed from the body by hemodialysis.
Angiotensin II could presumably serve as a specific antagonist-antidote in the setting of ramipril overdose, but angiotensin II is essentially unavailable outside of scattered research facilities. Because the hypotensive effect of ramipril is achieved through vasodilation and effective hypovolemia, it is reasonable to treat ramipril overdose by infusion of normal saline solution.
-
11 DESCRIPTION
Ramipril is an angiotensin converting enzyme (ACE) inhibitor. It is a white, crystalline substance soluble in polar organic solvents and buffered aqueous solutions. Ramipril melts between 105°C–112°C.
Ramipril’s chemical name is (2S,3aS,6aS)-1[(S)-N-[(S)-1-Carboxy-3- phenylpropyl] alanyl] octahydrocyclopenta [b]pyrrole-2-carboxylic acid, 1-ethyl ester.
The structural formula for ramipril is:
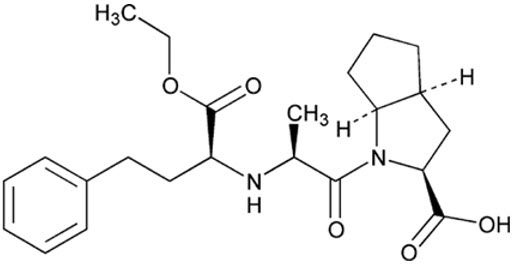
Its empirical formula is C 23H 32N 2O 5and its molecular weight is 416.5 g/mol.
VOSTALLY is a clear, colorless solution for oral use that contains 1 mg/mL ramipril. The inactive ingredients are citric acid monohydrate, ethylparaben sodium, frozen peppermint flavor 5015241T, methylparaben sodium, purified water, and sucralose.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ramipril and ramiprilat inhibit ACE in human subjects and animals. Angiotensin converting enzyme is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex. Inhibition of ACE results in decreased plasma angiotensin II, which leads to decreased vasopressor activity and to decreased aldosterone secretion. The latter decrease may result in a small increase of serum potassium. In hypertensive patients with normal renal function treated with ramipril alone for up to 56 weeks, approximately 4% of patients during the trial had an abnormally high serum potassium and an increase from baseline greater than 0.75 mEq/L, and none of the patients had an abnormally low potassium and a decrease from baseline greater than 0.75 mEq/L. In the same study, approximately 2% of patients treated with ramipril and hydrochlorothiazide for up to 56 weeks had abnormally high potassium values and an increase from baseline of 0.75 mEq/L or greater; and approximately 2% had abnormally low values and decreases from baseline of 0.75 mEq/L or greater [see Warnings and Precautions (5.8)] . Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity.
The effect of ramipril on hypertension appears to result at least in part from inhibition of both tissue and circulating ACE activity, thereby reducing angiotensin II formation in tissue and plasma.
Angiotensin converting enzyme is identical to kininase, an enzyme that degrades bradykinin. Whether increased levels of bradykinin, a potent vasopressor peptide, play a role in the therapeutic effects of VOSTALLY remains to be elucidated.
While the mechanism through which VOSTALLY lowers blood pressure is believed to be primarily suppression of the renin- angiotensin-aldosterone system, VOSTALLY has an antihypertensive effect even in patients with low-renin hypertension.
Although VOSTALLY was antihypertensive in all races studied, Black hypertensive patients (usually a low-renin hypertensive population) had a blood pressure lowering response to monotherapy, albeit a smaller average response, than non-Black patients.
12.2 Pharmacodynamics
Single doses of ramipril of 2.5 mg–20 mg produce approximately 60%–80% inhibition of ACE activity 4 hours after dosing with approximately 40%–60% inhibition after 24 hours. Multiple oral doses of ramipril of 2.5 mg or more cause plasma ACE activity to fall by more than 90% 4 hours after dosing, with over 80% inhibition of ACE activity remaining 24 hours after dosing. The more prolonged effect of even small multiple doses presumably reflects saturation of ACE binding sites by ramiprilat and relatively slow release from those sites.
12.3 Pharmacokinetics
Plasma concentrations of ramipril and ramiprilat (active metabolite) increase with increase in dose but are not strictly dose-proportional. The 24-hour AUC for ramiprilat, however, is dose-proportional over the 2.5 mg - 20 mg dose range. After once-daily dosing, steady-state plasma concentrations of ramiprilat are reached by the fourth dose. Steady-state concentrations of ramiprilat are somewhat higher than those seen after the first dose of ramipril, especially at low doses (2.5 mg), but the difference is not clinically significant plasma concentrations of ramiprilat decline in a triphasic manner (initial rapid decline, apparent elimination phase, terminal elimination phase).
Absorption
Following oral administration of VOSTALLY, peak plasma concentrations (Cmax) of ramipril are reached within 1 hour. Peak plasma concentrations of ramiprilat are reached 1–6 hours after drug intake. The absolute bioavailability of ramipril and ramiprilat were 28% and 44%, respectively, when 5 mg of oral ramipril was compared with the same dose of ramipril given intravenously.
Effect of food
The extent of absorption is not significantly influenced by the presence of food in the gastrointestinal tract, although the rate of absorption is reduced.
Distribution
The serum protein binding is about 73% for ramipril and about 56% for ramiprilat; in vitro, these percentages are independent of concentration over the range of 0.01 μg/mL–10 μg/mL The initial rapid decline, which represents distribution of the drug into a large peripheral compartment and subsequent binding to both plasma and tissue ACE, has a half-life of 2–4 hours.
Elimination
Because of its potent binding to ACE and slow dissociation from the enzyme, ramiprilat shows two elimination phases. The apparent elimination phase corresponds to the clearance of free ramiprilat and has a half-life of 9–18 hours. The terminal elimination phase has a prolonged half-life (>50 hours) and probably represents the binding/dissociation kinetics of the ramiprilat/ACE complex. It does not contribute to the accumulation of the drug. After multiple daily doses of ramipril 5 mg - 10 mg, the half-life of ramiprilat concentrations within the therapeutic range was 13–17 hours.
Metabolism
Cleavage of the ester group (primarily in the liver) converts ramipril to its active diacid metabolite, ramiprilat. Ramipril is almost completely metabolized to ramiprilat, which has about 6 times the ACE inhibitory activity of ramipril, and to the diketopiperazine ester, the diketopiperazine acid, and the glucuronides of ramipril and ramiprilat, all of which are inactive.
Excretion
After oral administration of ramipril, about 60% of the parent drug and its metabolites are eliminated in the urine, and about 40% is found in the feces. Drug recovered in the feces may represent both biliary excretion of metabolites and/or unabsorbed drug, however the proportion of a dose eliminated by the bile has not been determined. Less than 2% of the administered dose is recovered in urine as unchanged ramipril.
Specificpopulations
Patient with Renal Impairment
The urinary excretion of ramipril, ramiprilat, and their metabolites is reduced in patients with impaired renal function. Compared to normal subjects, patients with creatinine clearance <40 mL/min/1.73 m 2had higher peak and trough ramiprilat levels and slightly longer times to peak concentrations. In patients with creatinine clearance <40 mL/min/1.73 m 2, peak levels of ramiprilat are approximately doubled, and trough levels may be as much as quintupled. In multiple-dose regimens, the total exposure to ramiprilat (AUC) in these patients is 3–4 times greater than in patients with normal renal function who receive similar doses.
Patient with Hepatic Impairment
In patients with impaired liver function, the metabolism of ramipril to ramiprilat appears to be slowed, possibly because of diminished activity of hepatic esterases, and plasma ramipril levels in these patients are increased about 3-fold. Peak concentrations of ramiprilat in these patients, however, are not different from those seen in subjects with normal hepatic function, and the effect of a given dose on plasma ACE activity does not vary with hepatic function.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of a tumorigenic effect was found when ramipril was given by gavage to rats for up to 24 months at doses of up to 500 mg/kg/day or to mice for up to 18 months at doses of up to 1000 mg/kg/day. (For either species, these doses are about 200 times the maximum recommended human dose when compared on the basis of body surface area.) No mutagenic activity was detected in the Ames test in bacteria, the micronucleus test in mice, unscheduled DNA synthesis in a human cell line, or a forward gene-mutation assay in a Chinese hamster ovary cell line. Several metabolites and degradation products of ramipril were also negative in the Ames test. A study in rats with dosages as great as 500 mg/kg/day did not produce adverse effects on fertility.
No teratogenic effects of ramipril were seen in studies of pregnant rats, rabbits, and cynomolgus monkeys. On a body surface area basis, the doses used were up to approximately 400 times (in rats and monkeys) and 2 times (in rabbits) the recommended human dose.
-
14 CLINICAL STUDIES
14.1 Hypertension
Ramipril has been compared with other ACE inhibitors, beta-blockers, and thiazide diuretics as monotherapy for hypertension. It was approximately as effective as other ACE inhibitors and as atenolol.
Administration of VOSTALLY to patients with mild to moderate hypertension results in a reduction of both supine and standing blood pressure to about the same extent with no compensatory tachycardia. Symptomatic postural hypotension is infrequent, although it can occur in patients who are salt- and/or volume-depleted [see Warnings and Precautions (5.5)]. Use of VOSTALLY in combination with thiazide diuretics gives a blood pressure lowering effect greater than that seen with either agent alone.
In single-dose studies, doses of 5 mg–20 mg of ramipril lowered blood pressure within 1–2 hours, with peak reductions achieved 3–6 hours after dosing. The antihypertensive effect of a single dose persisted for 24 hours. In longer term (4–12 weeks) controlled studies, once-daily doses of 2.5 mg–10 mg were similar in their effect, lowering supine or standing systolic and diastolic blood pressures 24 hours after dosing by about 6/4 mmHg more than placebo. In comparisons of peak vs. trough effect, the trough effect represented about 50-60% of the peak response. In a titration study comparing divided bid vs. qd treatment, the divided regimen was superior, indicating that for some patients, the antihypertensive effect with once-daily dosing is not adequately maintained.
In most trials, the antihypertensive effect of ramipril increased during the first several weeks of repeated measurements. The antihypertensive effect of ramipril has been shown to continue during long-term therapy for at least 2 years. Abrupt withdrawal of ramipril has not resulted in a rapid increase in blood pressure. In both Caucasians and Blacks, hydrochlorothiazide (25 or 50 mg) was significantly more effective than ramipril.
Ramipril was less effective in blacks than in Caucasians. The effectiveness of ramipril was not influenced by age, sex, or weight.
In a baseline-controlled study of 10 patients with mild essential hypertension, blood pressure reduction was accompanied by a 15% increase in renal blood flow. In healthy volunteers, glomerular filtration rate was unchanged.
14.2 Reduction in Risk of Myocardial Infarction, Stroke, and Death from Cardiovascular Causes
The HOPE study was a large, multicenter, randomized, double-blind, placebo-controlled, 2 x 2 factorial design study conducted in 9541 patients (4645 on ramipril) who were 55 years or older and considered at high risk of developing a major cardiovascular event because of a history of coronary artery disease, stroke, peripheral vascular disease, or diabetes that was accompanied by at least one other cardiovascular risk factor (hypertension, elevated total cholesterol levels, low HDL levels, cigarette smoking, or documented microalbuminuria). Patients were either normotensive or under treatment with other antihypertensive agents. Patients were excluded if they had clinical heart failure or were known to have a low ejection fraction (<0.40). This study was designed to examine the long-term (mean of 5 years) effects of ramipril (10 mg orally once daily) on the combined endpoint of myocardial infarction, stroke, or death from cardiovascular causes.
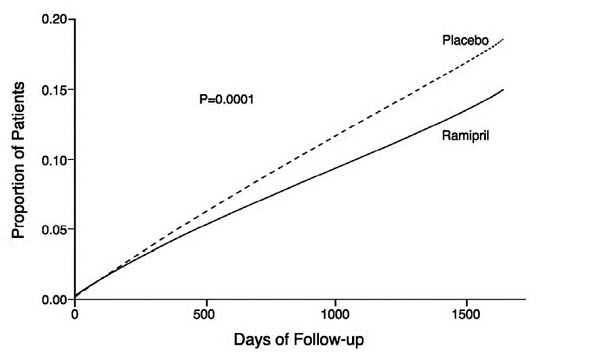
The HOPE study results showed that ramipril (10 mg/day) significantly reduced the rate of myocardial infarction, stroke, or death from cardiovascular causes (826/4652 vs. 651/4645, relative risk 0.78), as well as the rates of the 3 components of the combined endpoint. The relative risk of the composite outcomes in the ramipril group as compared to the placebo group was 0.78% (95% confidence interval, 0.70–0.86). The effect was evident after about 1 year of treatment.
Table 1. Summary of Combined Components and Endpoints—HOPE Study Outcome Placebo
(N=4652)
n (%)(ramipril)
(N=4645)
n (%)Relative Risk
(95% CI)
P-ValueCombined Endpoint Myocardial infarction, stroke, or death from cardiovascular cause 826 (17.8%) 651 (14.0%) 0.78 (0.70–0.86)
P=0.0001Component Endpoint Death from cardiovascular causes 377 (8.1%) 282 (6.1%) 0.74 (0.64–0.87)
P=0.0002Myocardial infarction 570 (12.3%) 459 (9.9%) 0.80 (0.70–0.90)
P=0.0003Stroke 226 (4.9%) 156 (3.4%) 0.68 (0.56–0.84)
P=0.0002Overall Mortality Death from any cause 569 (12.2%) 482 (10.4%) 0.84 (0.75–0.95)
P=0.005Figure 1. Kaplan-Meier Estimates of the Composite Outcome of Myocardial Infarction, Stroke, or Death from Cardiovascular Causes in the Ramipril Group and the Placebo Group
Ramipril was effective in different demographic subgroups (i.e., gender, age), subgroups defined by underlying disease (e.g., cardiovascular disease, hypertension), and subgroups defined by concomitant medication. There were insufficient data to determine whether or not ramipril was equally effective in ethnic subgroups.
This study was designed with a prespecified substudy in diabetics with at least one other cardiovascular risk factor. Effects of ramipril on the combined endpoint and its components were similar in diabetics (N=3577) to those in the overall study population.
Table 2. Summary of Combined Endpoints and Components in Diabetics—HOPE Study Outcome Placebo
(N=1769) n
(%)(ramipril)
(N=1808) n
(%)Relative Risk Reduction (95% CI)
P-ValueCombined Endpoint Myocardial infarction, stroke, or death from cardiovascular cause 351 (19.8%) 277 (15.3%) 0.25 (0.12–0.36)
P=0.0004Component Endpoint Death from cardiovascular causes 172 (9.7%) 112 (6.2%) 0.37 (0.21–0.51)
P=0.0001Myocardial infarction 229 (12.9%) 185 (10.2%) 0.22 (0.06–0.36)
P=0.01Stroke 108 (6.1%) 76 (4.2%) 0.33 (0.10–0.50)
P=0.007Figure 2. The Beneficial Effect of Treatment with ramipril on the Composite Outcome of Myocardial Infarction, Stroke, or Death from Cardiovascular Causes Overall and in Various Subgroups
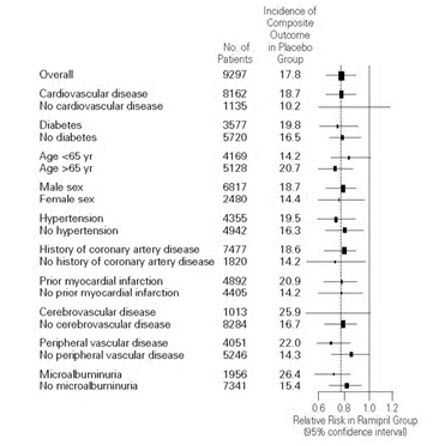
Cerebrovascular disease was defined as stroke or transient ischemic attacks. The size of each symbol is proportional to the number of patients in each group. The dashed line indicates overall relative risk.
The benefits of ramipril were observed among patients who were taking aspirin or other anti-platelet agents, beta- blockers, and lipid-lowering agents as well as diuretics and calcium channel blockers.
14.3 Post-Myocardial Infarction Heart Failure
Ramipril was studied in the AIRE trial. This was a multinational (mainly European) 161-center, 2006-patient, double- blind, randomized, parallel-group study comparing ramipril to placebo in stable patients, 2–9 days after an acute myocardial infarction, who had shown clinical signs of congestive heart failure at any time after the myocardial infarction. Patients in severe (NYHA class IV) heart failure, patients with unstable angina, patients with heart failure of congenital or valvular etiology, and patients with contraindications to ACE inhibitors were all excluded. The majority of patients had received thrombolytic therapy at the time of the index infarction, and the average time between infarction and initiation of treatment was 5 days.
Patients randomized to ramipril treatment were given an initial dose of 2.5 mg twice daily. If the initial regimen caused undue hypotension, the dose was reduced to 1.25 mg, but in either event doses were titrated upward (as tolerated) to a target regimen (achieved in 77% of patients randomized to ramipril) of 5 mg twice daily. Patients were then followed for an average of 15 months, with the range of follow-up between 6 and 46 months.
The use of ramipril was associated with a 27% reduction (p=0.002) in the risk of death from any cause; about 90% of the deaths that occurred were cardiovascular, mainly sudden death. The risks of progression to severe heart failure and of congestive heart failure-related hospitalization were also reduced, by 23% (p=0.017) and 26% (p=0.011), respectively.
The benefits of ramipril therapy were seen in both genders, and they were not affected by the exact timing of the initiation of therapy, but older patients may have had a greater benefit than those under 65. The benefits were seen in patients on (and not on) various concomitant medications. At the time of randomization these included aspirin (about 80% of patients), diuretics (about 60%), organic nitrates (about 55%), beta-blockers (about 20%), calcium channel blockers (about 15%), and digoxin (about 12%).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
VOSTALLY is a clear, colorless, solution that contains 150 mL of ramipril solution 1 mg/mL. It is supplied in a glass bottle with child-resistant cap (NDC: 69528-303-05).
-
17 PATIENT COUNSELING INFORMATION
Pregnancy
Advise female patients of reproductive potential about the consequences of exposure to VOSTALLY during pregnancy. Discuss treatment options with women planning to become pregnant. Ask patients to report pregnancies to their physicians as soon as possible [see Use in Specific Populations (8.1)].
Angioedema
Angioedema, including laryngeal edema, can occur with treatment with ACE inhibitors, especially following the first dose. Advise patients to immediately report any signs or symptoms suggesting angioedema (swelling of face, eyes, lips, or tongue, or difficulty in breathing) and to temporarily discontinue drug until they have consulted with the prescribing physician [see Warnings and Precautions (5.2)].
Symptomatic Hypotension
Inform patients that light-headedness can occur, especially during the first days of therapy, and it should be reported. Advise patients to discontinue VOSTALLY if syncope (fainting) occurs, and to follow up with their health care providers.
Inform patients that inadequate fluid intake or excessive perspiration, diarrhea, or vomiting while taking VOSTALLY can lead to an excessive fall in blood pressure, with the same consequences of lightheadedness and possible syncope [see Warnings and Precautions (5.3)].
Lactation
Advise women not to breastfeed during treatment with VOSTALLY [see Use in Specific Populations (8.2)].
Hyperkalemia
Advise patients not to use salt substitutes containing potassium without consulting their physician [see Warnings and Precautions (5.6)].
Hypoglycemia
Tell diabetic patients treated with oral antidiabetic agents or insulin starting an ACE inhibitor to monitor for hypoglycemia closely, especially during the first month of combined use [see Drug Interactions (7.2)].
Leukopenia/Neutropenia
Tell patients to report promptly any indication of infection (e.g., sore throat, fever), which may be a sign of leukopenia/neutropenia.
Administration Information
Instruct patients or caregivers to use an oral dosing syringe or oral dosing cup to correctly measure the prescribed amount of medication. Inform patients that oral dosing syringes may be obtained from their pharmacy.
-
SPL UNCLASSIFIED SECTION
Manufactured for:
Rosemont Pharmaceuticals LLC
Greenville, SC 29615
www.rosemontpharmaceuticals.com
VOSTALLY® is a registered trademark of Rosemont Pharmaceuticals Inc.
PI-RAM-01-001
- PRINCIPAL DISPLAY PANEL - 150 mL Bottle
-
INGREDIENTS AND APPEARANCE
VOSTALLY
ramipril solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69528-303 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RAMIPRIL (UNII: L35JN3I7SJ) (RAMIPRILAT - UNII:6N5U4QFC3G) RAMIPRIL 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength METHYLPARABEN (UNII: A2I8C7HI9T) 1.8 mg in 1 mL ETHYLPARABEN (UNII: 14255EXE39) SUCRALOSE (UNII: 96K6UQ3ZD4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PEPPERMINT (UNII: V95R5KMY2B) WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor PEPPERMINT (mint-flavored) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69528-303-05 1 in 1 CARTON 10/28/2025 1 150 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA219757 10/28/2025 Labeler - Metacel Pharmaceuticals, LLC (079475312)
Trademark Results [Vostally]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VOSTALLY 98200124 not registered Live/Pending |
Rosemont Pharmaceuticals Limited 2023-09-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
