These highlights do not include all the information needed to use SIMBRINZA safely and effectively. See full prescribing information for SIMBRINZA. SIMBRINZA® (brinzolamide and brimonidine tartrate ophthalmic suspension)Initial U.S. Approval: 2013
SIMBRINZA by
Drug Labeling and Warnings
SIMBRINZA by is a Prescription medication manufactured, distributed, or labeled by Novartis Pharmaceuticals Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SIMBRINZA- brinzolamide/brimonidine tartrate suspension/ drops
Novartis Pharmaceuticals Corporation
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SIMBRINZA safely and effectively. See full prescribing information for SIMBRINZA.
SIMBRINZA® (brinzolamide and brimonidine tartrate ophthalmic suspension) Initial U.S. Approval: 2013 INDICATIONS AND USAGESIMBRINZA is a fixed combination of a carbonic anhydrase inhibitor and an alpha-2 adrenergic receptor agonist indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. (1) DOSAGE AND ADMINISTRATIONShake well before use. Instill one drop in the affected eye(s) three times daily. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart. (2) DOSAGE FORMS AND STRENGTHSSuspension containing 10 mg/mL brinzolamide and 2 mg/mL brimonidine tartrate. (3) CONTRAINDICATIONSWARNINGS AND PRECAUTIONSADVERSE REACTIONSMost common adverse reactions occurring in approximately 3% to 5% of patients included blurred vision, eye irritation, dysgeusia (bad taste), dry mouth, and eye allergy. (6.1)
DRUG INTERACTIONSSee 17 for PATIENT COUNSELING INFORMATION. Revised: 11/2019 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
SIMBRINZA (brinzolamide and brimonidine tartrate ophthalmic suspension) 1% and 0.2% is a fixed combination of a carbonic anhydrase inhibitor and an alpha-2 adrenergic receptor agonist indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
2 DOSAGE AND ADMINISTRATION
The recommended dose is one drop of SIMBRINZA in the affected eye(s) three times daily. Shake well before use. SIMBRINZA ophthalmic suspension may be used concomitantly with other topical ophthalmic drug products to lower IOP. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart.
3 DOSAGE FORMS AND STRENGTHS
Suspension containing 10 mg/mL brinzolamide and 2 mg/mL brimonidine tartrate.
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Sulfonamide Hypersensitivity Reactions
SIMBRINZA contains brinzolamide, a sulfonamide, and although administered topically is absorbed systemically. Therefore, the same types of adverse reactions that are attributable to sulfonamides may occur with topical administration of SIMBRINZA. Fatalities have occurred due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias. Sensitization may recur when a sulfonamide is re-administered irrespective of the route of administration. If signs of serious reactions or hypersensitivity occur, discontinue the use of this preparation [see Patient Counseling Information (17)].
5.2 Corneal Endothelium
Carbonic anhydrase activity has been observed in both the cytoplasm and around the plasma membranes of the corneal endothelium. There is an increased potential for developing corneal edema in patients with low endothelial cell counts. Caution should be used when prescribing SIMBRINZA to this group of patients.
5.3 Severe Renal Impairment
SIMBRINZA has not been specifically studied in patients with severe renal impairment (CrCl less than 30 mL/min). Since brinzolamide and its metabolites are excreted predominantly by the kidney, SIMBRINZA is not recommended in such patients.
5.4 Acute Angle-Closure Glaucoma
The management of patients with acute angle-closure glaucoma requires therapeutic interventions in addition to ocular hypotensive agents. SIMBRINZA has not been studied in patients with acute angle-closure glaucoma.
5.5 Contact Lens Wear
The preservative in SIMBRINZA, benzalkonium chloride, may be absorbed by soft contact lenses. Contact lenses should be removed during instillation of SIMBRINZA but may be reinserted 15 minutes after instillation [see Patient Counseling Information (17)].
5.6 Severe Cardiovascular Disease
Brimonidine tartrate, a component of SIMBRINZA, has a less than 5% mean decrease in blood pressure two hours after dosing in clinical studies; caution should be exercised in treating patients with severe cardiovascular disease.
5.7 Severe Hepatic Impairment
Because brimonidine tartrate, a component of SIMBRINZA, has not been studied in patients with hepatic impairment, caution should be exercised in such patients.
5.8 Potentiation of Vascular Insufficiency
Brimonidine tartrate, a component of SIMBRINZA, may potentiate syndromes associated with vascular insufficiency. SIMBRINZA should be used with caution in patients with depression, cerebral or coronary insufficiency, Raynaud’s phenomenon, orthostatic hypotension, or thromboangiitis obliterans.
5.9 Contamination of Topical Ophthalmic Products After Use
There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers have been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface [see Patient Counseling Information (17)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
SIMBRINZA
In two clinical trials of three months duration 435 patients were treated with SIMBRINZA, and 915 were treated with the two individual components. The most frequently reported adverse reactions in patients treated with SIMBRINZA occurring in approximately 3% to 5% of patients in descending order of incidence were blurred vision, eye irritation, dysgeusia (bad taste), dry mouth, and eye allergy. Rates of adverse reactions reported with the individual components were comparable. Treatment discontinuation, mainly due to adverse reactions, was reported in 11% of SIMBRINZA patients.
Other adverse reactions that have been reported with the individual components during clinical trials are listed below.
Brinzolamide 1%
In clinical trials of brinzolamide ophthalmic suspension 1%, the most frequently reported adverse reactions reported in 5% to 10% of patients were blurred vision and bitter, sour or unusual taste. Adverse reactions occurring in 1% to 5% of patients were blepharitis, dermatitis, dry eye, foreign body sensation, headache, hyperemia, ocular discharge, ocular discomfort, ocular keratitis, ocular pain, ocular pruritus, and rhinitis.
The following adverse reactions were reported at an incidence below 1%: allergic reactions, alopecia, chest pain, conjunctivitis, diarrhea, diplopia, dizziness, dry mouth, dyspnea, dyspepsia, eye fatigue, hypertonia, keratoconjunctivitis, keratopathy, kidney pain, lid margin crusting or sticky sensation, nausea, pharyngitis, tearing, and urticaria.
Brimonidine Tartrate 0.2%
In clinical trials of brimonidine tartrate 0.2%, adverse reactions occurring in approximately 10% to 30% of the subjects, in descending order of incidence, included oral dryness, ocular hyperemia, burning and stinging, headache, blurring, foreign body sensation, fatigue/drowsiness, conjunctival follicles, ocular allergic reactions, and ocular pruritus.
Reactions occurring in approximately 3% to 9% of the subjects, in descending order included corneal staining/erosion, photophobia, eyelid erythema, ocular ache/pain, ocular dryness, tearing, upper respiratory symptoms, eyelid edema, conjunctival edema, dizziness, blepharitis, ocular irritation, gastrointestinal symptoms, asthenia, conjunctival blanching, abnormal vision, and muscular pain.
The following adverse reactions were reported in less than 3% of the patients: lid crusting, conjunctival hemorrhage, abnormal taste, insomnia, conjunctival discharge, depression, hypertension, anxiety, palpitations/arrhythmias, nasal dryness, and syncope.
6.2 Postmarketing Experience
The following reactions have been identified during postmarketing use of brimonidine tartrate ophthalmic solutions in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to brimonidine tartrate ophthalmic solutions, or a combination of these factors, include: bradycardia, hypersensitivity, iritis, keratoconjunctivitis sicca, miosis, nausea, skin reactions (including erythema, eyelid pruritus, rash, and vasodilation), and tachycardia.
Apnea, bradycardia, coma, hypotension, hypothermia, hypotonia, lethargy, pallor, respiratory depression, and somnolence have been reported in infants receiving brimonidine tartrate ophthalmic solutions [see Contraindications (4.2)].
7 DRUG INTERACTIONS
7.1 Oral Carbonic Anhydrase Inhibitors
There is a potential for an additive effect on the known systemic effects of carbonic anhydrase inhibition in patients receiving an oral carbonic anhydrase inhibitor and brinzolamide ophthalmic suspension 1%, a component of SIMBRINZA ophthalmic suspension. The concomitant administration of SIMBRINZA and oral carbonic anhydrase inhibitors is not recommended.
7.2 High-Dose Salicylate Therapy
Carbonic anhydrase inhibitors may produce acid-base and electrolyte alterations. These alterations were not reported in the clinical trials with brinzolamide ophthalmic suspension 1%. However, in patients treated with oral carbonic anhydrase inhibitors, rare instances of acid-base alterations have occurred with high-dose salicylate therapy. Therefore, the potential for such drug interactions should be considered in patients receiving SIMBRINZA.
7.3 Central Nervous System Depressants
Although specific drug interaction studies have not been conducted with SIMBRINZA, the possibility of an additive or potentiating effect with central nervous system (CNS) depressants (alcohol, opiates, barbiturates, sedatives, or anesthetics) should be considered.
7.4 Antihypertensives/Cardiac Glycosides
Because brimonidine tartrate, a component of SIMBRINZA, may reduce blood pressure, caution in using drugs such as antihypertensives and/or cardiac glycosides with SIMBRINZA is advised.
7.5 Tricyclic Antidepressants
Tricyclic antidepressants have been reported to blunt the hypotensive effect of systemic clonidine. It is not known whether the concurrent use of these agents with SIMBRINZA in humans can lead to resulting interference with the IOP lowering effect. Caution is advised in patients taking tricyclic antidepressants, which can affect the metabolism and uptake of circulating amines.
7.6 Monoamine Oxidase Inhibitors
Monoamine oxidase inhibitors (MAOIs) may theoretically interfere with the metabolism of brimonidine tartrate and potentially result in an increased systemic side-effect such as hypotension. Caution is advised in patients taking MAOIs, which can affect the metabolism and uptake of circulating amines.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C: Developmental toxicity studies with brinzolamide in rabbits at oral doses of 1, 3, and 6 mg/kg/day (20, 60, and 120 times the recommended human ophthalmic dose) produced maternal toxicity at 6 mg/kg/day and a significant increase in the number of fetal variations, such as accessory skull bones, which was only slightly higher than the historic value at 1 and 6 mg/kg. In rats, statistically decreased body weights of fetuses from dams receiving oral doses of 18 mg/kg/day (180 times the recommended human ophthalmic dose) during gestation were proportional to the reduced maternal weight gain, with no statistically significant effects on organ or tissue development. Increases in unossified sternebrae, reduced ossification of the skull, and unossified hyoid that occurred at 6 and 18 mg/kg were not statistically significant. No treatment-related malformations were seen. Following oral administration of 14C-brinzolamide to pregnant rats, radioactivity was found to cross the placenta and was present in the fetal tissues and blood.
Developmental toxicity studies performed in rats with oral doses of 0.66 mg brimonidine base/kg revealed no evidence of harm to the fetus. Dosing at this level resulted in a plasma drug concentration approximately 100 times higher than that seen in humans at the recommended human ophthalmic dose. In animal studies, brimonidine crossed the placenta and entered into the fetal circulation to a limited extent.
There are no adequate and well-controlled studies in pregnant women. SIMBRINZA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
In a study of brinzolamide in lactating rats, decreases in body weight gain in offspring at an oral dose of 15 mg/kg/day (150 times the recommended human ophthalmic dose) were observed during lactation. No other effects were observed. However, following oral administration of 14C-brinzolamide to lactating rats, radioactivity was found in milk at concentrations below those in the blood and plasma. In animal studies, brimonidine was excreted in breast milk.
It is not known whether brinzolamide and brimonidine tartrate are excreted in human milk following topical ocular administration. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from SIMBRINZA, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The individual component, brinzolamide, has been studied in pediatric glaucoma patients 4 weeks to 5 years of age. The individual component, brimonidine tartrate, has been studied in pediatric patients two to seven years old. Somnolence (50% to 83%) and decreased alertness was seen in patients two to six years old. SIMBRINZA is contraindicated in children under the age of two years [see Contraindications (4.2)].
10 OVERDOSAGE
Although no human data are available, electrolyte imbalance, development of an acidotic state, and possible nervous system effects may occur following an oral overdose of brinzolamide. Serum electrolyte levels (particularly potassium) and blood pH levels should be monitored.
Very limited information exists on accidental ingestion of brimonidine in adults; the only adverse event reported to date has been hypotension. Symptoms of brimonidine overdose have been reported in neonates, infants, and children receiving brimonidine as part of medical treatment of congenital glaucoma or by accidental oral ingestion. Treatment of an oral overdose includes supportive and symptomatic therapy; a patent airway should be maintained.
11 DESCRIPTION
SIMBRINZA is a fixed combination of a carbonic anhydrase inhibitor and an alpha-2 adrenergic receptor agonist.
Brinzolamide is described chemically as: (R)-(+)-4-Ethylamino-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno [3,2-e]-1,2-thiazine-6-sulfonamide-1,1- dioxide. Its empirical formula is C12H21N3O5S3, and its structural formula is:
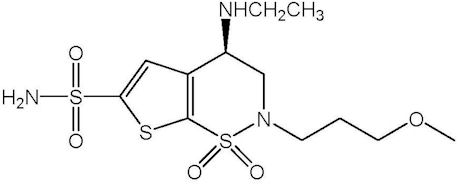
Brinzolamide has a molecular weight of 383.5 g/mol. It is a white powder, which is insoluble in water, very soluble in methanol and soluble in ethanol.
Brimonidine tartrate is described chemically as: 5-bromo-6-(2-imidazolidinylideneamino) quinoxaline L-tartrate. Its empirical formula of C11H10BrN5 – C4H6O6 and its structural formula is:
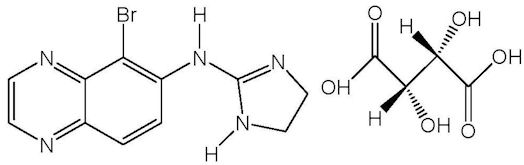
Brimonidine tartrate has a molecular weight of 442.2 g/mol. It is a white to yellow powder that is soluble in water (34 mg/mL) at pH 6.5.
SIMBRINZA is supplied as a sterile, aqueous suspension which has been formulated to be readily suspended following shaking. It has a pH of approximately 6.5 and an osmolality of approximately 270 mOsm/kg.
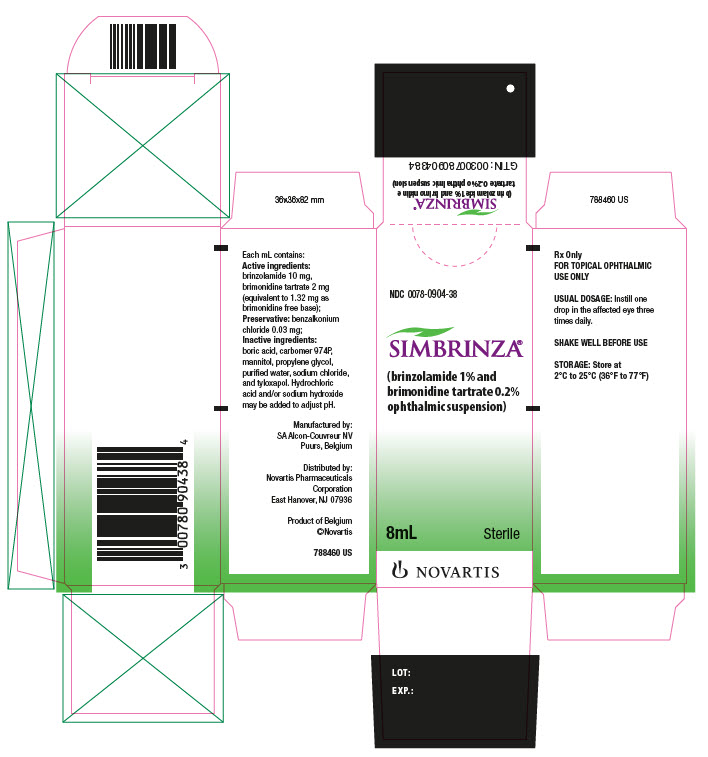
Each mL of SIMBRINZA contains: Active ingredients: brinzolamide 10 mg, brimonidine tartrate 2 mg (equivalent to 1.32 mg as brimonidine free base); Preservative: benzalkonium chloride 0.03 mg; Inactive ingredients: boric acid, carbomer 974P, mannitol, propylene glycol, purified water, sodium chloride, and tyloxapol. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
SIMBRINZA is comprised of two components: brinzolamide (carbonic anhydrase inhibitor) and brimonidine tartrate (alpha-2 adrenergic receptor agonist). Each of these two components decreases elevated IOP. Elevated IOP is a major risk factor in the pathogenesis of optic nerve damage and glaucomatous visual field loss. The higher the level of IOP, the greater the likelihood of glaucomatous field loss and optic nerve damage.
Brinzolamide inhibits carbonic anhydrase in the ciliary processes of the eye to decrease aqueous humor secretion, presumably by slowing the formation of bicarbonate ions with subsequent reduction in sodium and fluid transport. Brinzolamide has a peak ocular hypotensive effect occurring at two to three hours post-dosing. Fluorophotometric studies in animals and humans suggest that brimonidine tartrate has a dual mechanism of action by reducing aqueous humor production and increasing uveoscleral outflow. Brimonidine tartrate has a peak ocular hypotensive effect occurring at two hours post-dosing. The result is a reduction in IOP.
12.3 Pharmacokinetics
Following topical ocular administration, brinzolamide is absorbed into the systemic circulation. Due to its affinity for CA-II, brinzolamide distributes extensively into the red blood cells (RBCs) and exhibits a long half-life in whole blood (approximately 111 days). In humans, the metabolite N-desethyl brinzolamide is formed, which also binds to CA and accumulates in RBCs. This metabolite binds mainly to CA-I in the presence of brinzolamide. In plasma, both parent brinzolamide and N-desethyl brinzolamide concentrations are less than 10 ng/mL. Binding to plasma proteins is approximately 60%. Brinzolamide is eliminated predominantly in the urine as unchanged drug. N-Desethyl brinzolamide is also found in the urine along with lower concentrations of the N-desmethoxypropyl and O-desmethyl metabolites.
After ocular administration of a 0.2% solution of brimonidine tartrate, plasma concentrations peaked within one to four hours and declined with a systemic half-life of approximately three hours. In humans, systemic metabolism of brimonidine is extensive. It is metabolized primarily by the liver. Urinary excretion is the major route of elimination of the drug and its metabolites. Approximately 87% of an orally-administered radioactive dose was eliminated within 120 hours, with 74% found in the urine.
In humans, a study was conducted to evaluate the pharmacokinetics of the fixed combination of brinzolamide and brimonidine tartrate 1% and 0.2% ophthalmic suspension. Healthy volunteers were randomly assigned to receive twice or three times a day either the fixed combination, or either of its individual components, brinzolamide or brimonidine. Subjects who were assigned to the brinzolamide alone or combination arms were administered oral brinzolamide capsules for two weeks prior to beginning dosing with the topical ocular suspension. The results demonstrate that the systemic plasma exposure (area under the curve [AUC)] and Cmax) to brinzolamide and brimonidine in humans is similar after dosing with the fixed combination to that observed following dosing with the individual components.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Brinzolamide caused urinary bladder tumors in female mice at oral doses of 10 mg/kg/day and in male rats at oral doses of 8 mg/kg/day in two year studies. Brinzolamide was not carcinogenic in male mice or female rats dosed orally for up to two years. The carcinogenicity appears secondary to kidney and urinary bladder toxicity. These levels of exposure cannot be achieved with topical ophthalmic dosing in humans.
The following tests for mutagenic potential of brinzolamide were negative: (1) in vivo mouse micronucleus assay; (2) in vivo sister chromatid exchange assay; and (3) Ames E. coli test. The in vitro mouse lymphoma forward mutation assay was negative in the absence of activation, but positive in the presence of microsomal activation. In this assay, there was no consistent dose-response relationship to the increased mutation frequency and cytotoxicity likely contributed to the high mutation frequency. Carbonic anhydrase inhibitors, as a class, are not mutagenic and the weight of evidence supports that brinzolamide is consistent with the class. In reproduction studies of brinzolamide in rats, there were no adverse effects on the fertility or reproductive capacity of males or females at doses up to 18 mg/kg/day (180 times the recommended human ophthalmic dose).
Brimonidine tartrate was not carcinogenic in either a 21-month mouse or 24-month rat study. In these studies, dietary administration of brimonidine tartrate at doses up to 2.5 mg/kg/day in mice and 1 mg/kg/day in rats resulted in plasma drug concentrations 80 and 120 times higher than the human plasma drug level at the recommended clinical dose, respectively. Brimonidine tartrate was not mutagenic or cytogenic in a series of in vitro and in vivo studies including the Ames test, chromosomal aberration assay in Chinese Hamster Ovary (CHO) cells, a host-mediated assay and cytogenic studies in mice, and a dominant lethal assay. In reproductive studies performed in rats with oral doses of 0.66 mg brimonidine base/kg (approximately 100 times the plasma drug concentration level seen in humans following multiple ophthalmic doses), fertility was not impaired.
14 CLINICAL STUDIES
Two clinical trials of three months duration were conducted in patients with open-angle glaucoma or ocular hypertension to compare the IOP-lowering effect of SIMBRINZA dosed three times daily to individually administered 1% brinzolamide three times daily and 0.2% brimonidine tartrate three times daily. Mean IOP values at baseline are presented in Table 1.
| Abbreviation: SD, standard deviation. | ||||
| SIMBRINZA | Brinzolamide | Brimonidine | ||
| Study 1 | (n = 209) | (n = 224) | (n = 216) | |
| 8 AM | 26.9 (2.63) | 27.1 (2.64) | 27.0 (2.56) | |
| 10 AM | 25.3 (2.76) | 25.4 (2.74) | 25.4 (2.78) | |
| 3 PM | 23.7 (2.98) | 23.8 (3.24) | 24.0 (3.27) | |
| 5 PM | 23.2 (3.08) | 23.6 (3.39) | 23.7 (3.30) | |
| Study 2 | (n = 218) | (n = 229) | (n = 232) | |
| 8 AM | 27.2 (2.75) | 27.2 (2.72) | 27.3 (2.73) | |
| 10 AM | 25.8 (3.09) | 26.0 (3.20) | 25.8 (3.02) | |
| 3 PM | 24.4 (3.67) | 24.4 (3.58) | 24.0 (3.39) | |
| 5 PM | 24.1 (3.71) | 24.2 (3.86) | 23.7 (3.58) | |
The IOP-lowering effect of SIMBRINZA ophthalmic suspension was 1 to 3 mmHg greater than monotherapy with either 1% brinzolamide or 0.2% brimonidine tartrate throughout the duration of the trials. Least Square Mean IOP (mmHg) and the results at Week 2, Week 6, and Month 3 for each study are provided in Table 2.
| Abbreviations: CI, confidence interval (95% CI); IOP, intraocular pressure. aBased on the Intent-to-Treat Population defined as all patients who received study drug and completed at least 1 on-therapy study visit. bThe estimates are based on least square means derived from a linear mixed model that accounts for correlated IOP measurements within patient; Treatment difference is SIMBRINZA minus individual component. |
|||||
| SIMBRINZA | Brinzolamide | Brimonidine | |||
| Study 1 | (N = 209) | (N = 224) | (N = 216) | ||
| Mean | Mean | Difference (95% CI)b | Mean | Difference (95% CI)b | |
| Week 2 | |||||
| 8 AM | 20.4 | 22.0 | -1.6 (-2.3, -0.9) | 22.4 | -2.0 (-2.7, -1.3) |
| 10 AM | 17.1 | 20.5 | -3.4 (-4.1, -2.7) | 19.4 | -2.3 (-3.0, -1.6) |
| 3 PM | 18.4 | 20.4 | -1.9 (-2.6, -1.3) | 20.6 | -2.2 (-2.9, -1.5) |
| 5 PM | 16.6 | 19.7 | -3.2 (-3.9, -2.5) | 18.4 | -1.9 (-2.6, -1.2) |
| Week 6 | |||||
| 8 AM | 20.4 | 21.9 | -1.5 (-2.2, -0.8) | 22.6 | -2.3 (-3.0, -1.6) |
| 10 AM | 17.5 | 20.2 | -2.7 (-3.4, -2.0) | 19.5 | -2.0 (-2.7, -1.3) |
| 3 PM | 18.9 | 20.2 | -1.2 (-1.9, -0.5) | 21.1 | -2.1 (-2.8, -1.4) |
| 5 PM | 17.0 | 19.7 | -2.6 (-3.3, -1.9) | 18.6 | -1.5 (-2.2, -0.8) |
| Month 3 | |||||
| 8 AM | 20.5 | 21.6 | -1.1 (-1.8, -0.4) | 23.3 | -2.8 (-3.5, -2.1) |
| 10 AM | 17.2 | 20.4 | -3.2 (-3.9, -2.5) | 19.7 | -2.5 (-3.2, -1.8) |
| 3 PM | 18.7 | 20.4 | -1.8 (-2.5, -1.1) | 21.3 | -2.6 (-3.3, -1.9) |
| 5 PM | 17.0 | 20.0 | -3.0 (-3.7, -2.3) | 18.8 | -1.8 (-2.5, -1.1) |
| Study 2 | (N = 218) | (N = 229) | (N = 232) | ||
| Week 2 | |||||
| 8 AM | 20.5 | 22.2 | -1.7 (-2.4, -1.0) | 22.8 | -2.4 (-3.1, -1.7) |
| 10 AM | 17.4 | 20.7 | -3.3 (-4.0, -2.6) | 19.2 | -1.8 (-2.5, -1.2) |
| 3 PM | 18.7 | 20.5 | -1.7 (-2.4, -1.1) | 21.1 | -2.3 (-3.0, -1.6) |
| 5 PM | 16.5 | 20.1 | -3.6 (-4.3, -2.9) | 18.3 | -1.8 (-2.4, -1.1) |
| Week 6 | |||||
| 8 AM | 20.7 | 21.9 | -1.2 (-1.9, -0.5) | 23.2 | -2.5 (-3.2, -1.8) |
| 10 AM | 17.4 | 20.5 | -3.1 (-3.8, -2.4) | 19.7 | -2.3 (-3.0, -1.6) |
| 3 PM | 19.3 | 20.2 | -0.8 (-1.5, -0.2) | 21.2 | -1.9 (-2.6, -1.2) |
| 5 PM | 16.9 | 19.9 | -3.0 (-3.7, -2.3) | 18.5 | -1.7 (-2.4, -1.0) |
| Month 3 | |||||
| 8 AM | 21.1 | 22.0 | -1.0 (-1.7, -0.3) | 23.2 | -2.2 (-2.9, -1.5) |
| 10 AM | 18.0 | 20.8 | -2.8 (-3.5, -2.1) | 19.9 | -1.9 (-2.6, -1.2) |
| 3 PM | 19.5 | 20.7 | -1.2 (-1.9, -0.5) | 21.5 | -2.0 (-2.7, -1.3) |
| 5 PM | 17.2 | 20.4 | -3.2 (-3.9, -2.5) | 18.9 | -1.7 (-2.4, -1.0) |
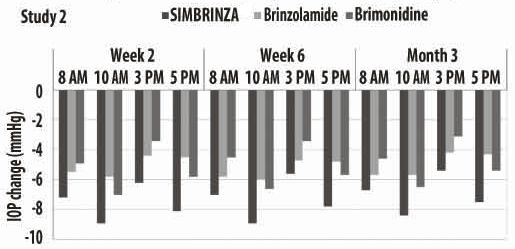
Figures 1 and 2 present the mean of individual subject IOP changes from baseline at Week 2, Week 6, and at Month 3 based on the observed data for the intent-to-treat population.
Figure 1. Mean IOP Change from Baseline (Study 1)
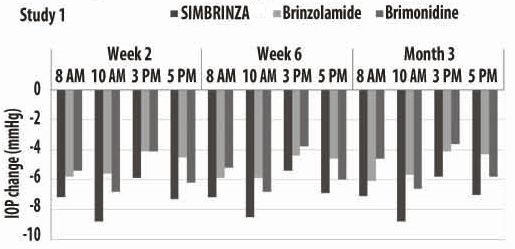
Figure 2. Mean IOP Change from Baseline (Study 2)
16 HOW SUPPLIED/STORAGE AND HANDLING
SIMBRINZA is supplied in white low density polyethylene (LDPE) DROP-TAINER® bottles with a natural LDPE dispensing-tip and light green polypropylene cap as follows:
8 mL in a 10 mL bottle NDC: 0078-0904-38
Storage and Handling
Store SIMBRINZA at 2°C to 25°C (36 to 77°F).
17 PATIENT COUNSELING INFORMATION
Sulfonamide Reactions
Advise patients that if serious or unusual ocular or systemic reactions or signs of hypersensitivity occur, they should discontinue the use of the product and consult their physician [see Warnings and Precautions (5.1)].
Temporary Blurred Vision
Vision may be temporarily blurred following dosing with SIMBRINZA. Care should be exercised in operating machinery or driving a motor vehicle.
Effect on Ability to Drive and Use Machinery
As with other drugs in this class, SIMBRINZA may cause fatigue and/or drowsiness in some patients. Caution patients who engage in hazardous activities of the potential for a decrease in mental alertness.
Avoiding Contamination of the Product
Instruct patients that ocular solutions, if handled improperly or if the tip of the dispensing container contacts the eye or surrounding structures, can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions [see Warnings and Precautions (5.9)]. Always replace the cap after using. If solution changes color or becomes cloudy, do not use. Do not use the product after the expiration date marked on the bottle.
Intercurrent Ocular Conditions
Advise patients that if they have ocular surgery or develop an intercurrent ocular condition (e.g., trauma or infection), they should immediately seek their physician's advice concerning the continued use of the present multidose container.
Concomitant Topical Ocular Therapy
If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart [see Dosage and Administration].
Contact Lens Wear
The preservative in SIMBRINZA, benzalkonium chloride, may be absorbed by soft contact lenses. Contact lenses should be removed during instillation of SIMBRINZA, but may be reinserted 15 minutes after instillation.
DROP-TAINER is a trademark of Alcon.
©Novartis
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, NJ 07936
T2019-124
PRINCIPAL DISPLAY PANEL
NDC: 0078-0904-38
SIMBRINZA®
(brinzolamide 1% and brimonidine tartrate 0.2% ophthalmic suspension)
8 mL
Sterile
Rx Only
FOR TOPICAL OPHTHALMIC USE ONLY
NOVARTIS
| SIMBRINZA
brinzolamide/brimonidine tartrate suspension/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |
Trademark Results [SIMBRINZA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SIMBRINZA 85306875 4388553 Live/Registered |
Novartis AG 2011-04-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.