ZICAM- oxymetazoline hydrochloride spray
Zicam by
Drug Labeling and Warnings
Zicam by is a Otc medication manufactured, distributed, or labeled by Matrixx Initiatives, Inc., Accupac, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
When using this product
- do not use more than directed
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may result
- use of this container by more than one person may spread infection
-
Directions
- adults and children 6 to under 12 years of age (with adult supervision): 2 or 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- children under 6 years of age: ask a doctor
To use pump:
- to open, hold the actuator to squeeze and turn cap
- hold with thumb at bottom of bottle and nozzle between fingers
- before using the first time, prime pump by depressing several times
- place tip of nozzle just past nasal opening (approximately 1/8")
- pump 2 or 3 times in each nostril without tilting your head. Sniff deeply.
- wipe nozzle clean after use
- to close, turn cap until it 'clicks'
- Other information
- Inactive ingredients
- Questions? Comments? Side Effects?
- SPL UNCLASSIFIED SECTION
-
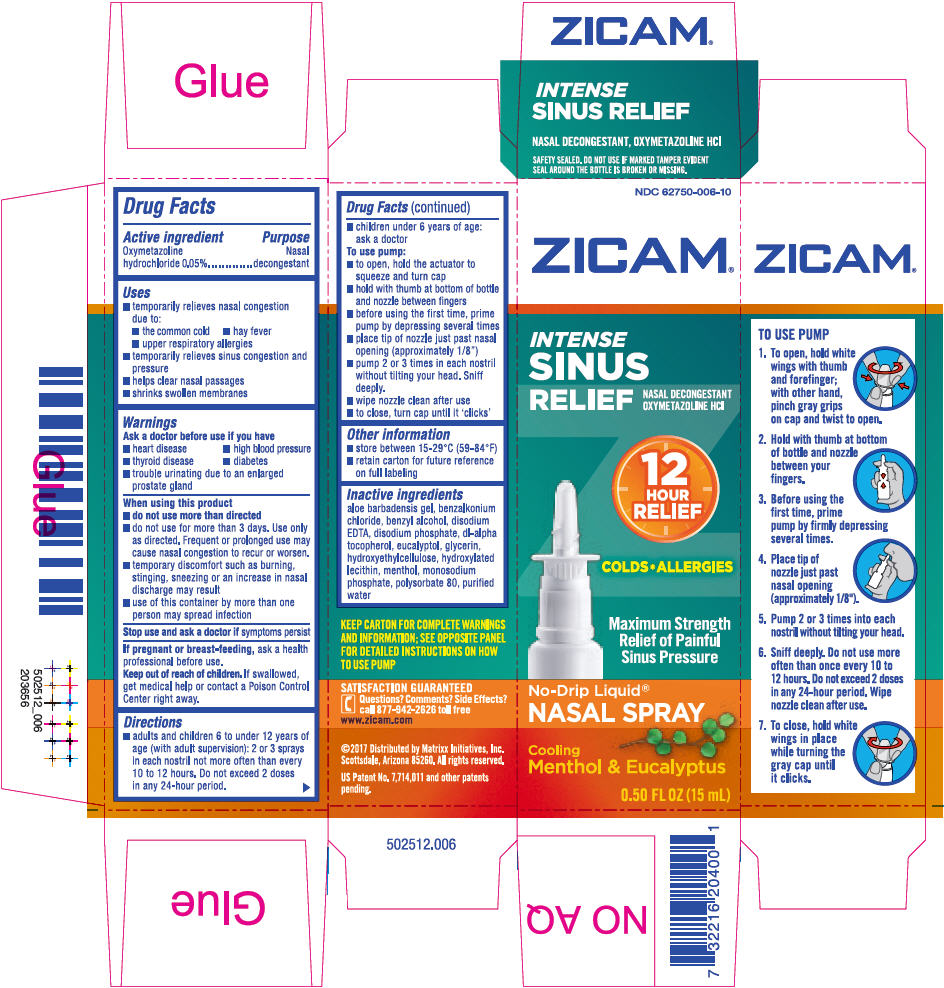
PRINCIPAL DISPLAY PANEL - 15 mL Bottle Carton
NDC: 62750-006-10
ZICAM®
INTENSE
SINUS
RELIEFNASAL DECONGESTANT
OXYMETAZOLINE HCl12
HOUR
RELIEFCOLDS*ALLERGIES
Maximum Strength
Relief of Painful
Sinus PressureNo-Drip Liquid®
NASAL SPRAYCooling
Menthol & Eucalyptus0.50 FL OZ (15 mL)
-
INGREDIENTS AND APPEARANCE
ZICAM
oxymetazoline hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62750-006 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength oxymetazoline hydrochloride (UNII: K89MJ0S5VY) (oxymetazoline - UNII:8VLN5B44ZY) oxymetazoline hydrochloride .5 mg in 1 mL Inactive Ingredients Ingredient Name Strength aloe vera leaf (UNII: ZY81Z83H0X) benzalkonium chloride (UNII: F5UM2KM3W7) benzyl alcohol (UNII: LKG8494WBH) edetate disodium (UNII: 7FLD91C86K) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) eucalyptol (UNII: RV6J6604TK) glycerin (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) polysorbate 80 (UNII: 6OZP39ZG8H) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62750-006-10 1 in 1 CARTON 05/29/2002 1 15 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 05/29/2002 Labeler - Matrixx Initiatives, Inc. (790037253) Establishment Name Address ID/FEI Business Operations Accupac, Inc. 071609663 MANUFACTURE(62750-006)
Trademark Results [Zicam]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZICAM 86709312 4913192 Live/Registered |
Zicam, LLC 2015-07-30 |
 ZICAM 76261513 2517404 Live/Registered |
ZICAM, LLC 2001-05-22 |
 ZICAM 75878475 2393752 Dead/Cancelled |
JAI PULNIX, INC. 1999-12-21 |
 ZICAM 75570566 not registered Dead/Abandoned |
Gel Tech Industries 1998-10-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.