Ketamine HCl Injection, USP
Ketamine Hydrochloride by
Drug Labeling and Warnings
Ketamine Hydrochloride by is a Animal medication manufactured, distributed, or labeled by Putney, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
KETAMINE HYDROCHLORIDE- ketamine hydrochloride injection
Putney, Inc.
----------
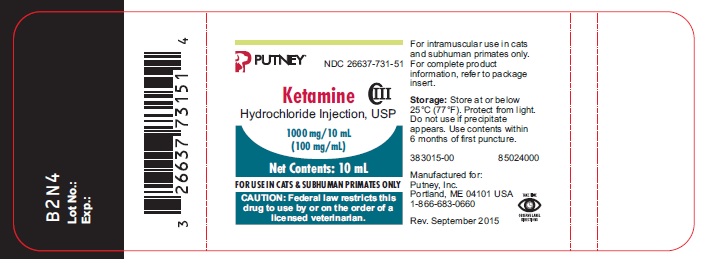
Ketamine HCl Injection, USP
NADA 045-290, Approved by FDA
Veterinary Injection for Intramuscular Use in Cats and Subhuman Primates Only
Description:
Ketamine Hydrochloride Injection, USP is a rapid-acting, nonnarcotic, nonbarbiturate agent for anesthetic use in cats and for restraint in subhuman primates. It is chemically designated dl 2-(o-chlorophenyl)-2-(methylamino) cyclohexanone hydrochloride and is supplied as a slightly acid (pH 3.5 to 5.5) solution for intramuscular injection in a concentration containing the equivalent of 100 mg ketamine base per milliliter and contains not more than 0.1 mg/mL benzethonium chloride as a preservative.
Indications:
Ketamine Hydrochloride Injection, USP may be used in cats for restraint or as the sole anesthetic agent for diagnostic or minor, brief, surgical procedures that do not require skeletal muscle relaxation. It may be used in subhuman primates for restraint.
Dosage and Administration:
Ketamine Hydrochloride Injection, USP is well tolerated by cats and subhuman primates when administered by intramuscular injection.
Fasting prior to induction of anesthesia or restraint with Ketamine Hydrochloride Injection, USP is not essential; however, when preparing for elective surgery, it is advisable to withhold food for at least six hours prior to administration of Ketamine Hydrochloride Injection, USP.
Anesthesia may be of shorter duration in immature cats. Restraint in subhuman primate neonates (less than 24 hours of age) is difficult to achieve.
As with other anesthetic agents, the individual response to Ketamine Hydrochloride Injection, USP is somewhat varied depending upon the dose, general condition and age of the subject so that dosage recommendations cannot be absolutely fixed.
Dosage – Cats:
A dose of 11 mg/kg (5 mg/lb) is recommended to produce restraint. Dosages from 22 to 33 mg/kg (10 to 15 mg/lb) produce anesthesia that is suitable for diagnostic or minor surgical procedures that do not require skeletal muscle relaxation.
Subhuman Primates:
The recommended restraint dosages Ketamine Hydrochloride Injection, USP for the following species are: Cercocebus torquatus (white-collared mangabey), Papio cynocephalus (yellow baboon), Pan troglodytes verus (chimpanzee), Papio anubis (olive baboon), Pongo pygmaeus (orangutan), Macaca nemestrina (pig-tailed macaque) 5 to 7.5 mg/kg; Presbytis entellus (entellus langur) 3 to 5 mg/kg; Gorilla gorilla gorilla (gorilla) 7 to 10 mg/kg; Aotus trivirgatus (night monkey) 10 to 12 mg/kg; Macaca mulatta (rhesus monkey) 5 to 10 mg/kg; Cebus capucinus (white-throated capuchin) 13 to 15 mg/kg; and Macaca fascicularis (crab-eating macaque), Macaca radiata (bonnet macaque) and Saimiri sciureus (squirrel monkey) 12 to 15 mg/kg.
A single intramuscular injection produces restraint suitable for TB testing; radiography, physical examination or blood collection.
Contraindications:
Ketamine Hydrochloride Injection, USP is contraindicated in cats and subhuman primates suffering from renal or hepatic insufficiency.
Ketamine Hydrochloride Injection, USP is detoxified by the liver and excreted by the kidneys; therefore, any preexistent hepatic or renal pathology or impairment of function can be expected to result in prolonged anesthesia; related fatalities have been reported.
Precautions:
In cats, doses in excess of 50 mg/kg during any single procedure should not be used. The maximum recommended dose in subhuman primates is 40 mg/kg.
To reduce the incidence of emergence reactions, animals should not be stimulated by sound or handling during the recovery period. However, this does not preclude the monitoring of vital signs.
Apnea, respiratory arrest, cardiac arrest and death have occasionally been reported with ketamine used alone, and more frequently when used in conjunction with sedatives or other anesthetics. Close monitoring of patients is strongly advised during induction, maintenance and recovery from anesthesia.
Color of solution may vary from colorless to very slightly yellowish and may darken upon prolonged exposure to light. This darkening does not affect potency. Do not use if precipitate appears.
Adverse Reactions:
Respiratory depression may occur following administration of high doses of Ketamine Hydrochloride Injection, USP. If at any time respiration becomes excessively depressed and the animal becomes cyanotic, resuscitative measures should be instituted promptly. Adequate pulmonary ventilation using either oxygen or room air is recommended as a resuscitative measure.
Adverse reactions reported have included emesis, salivation, vocalization, erratic recovery and prolonged recovery, spastic jerking movements, convulsions, muscular tremors, hypertonicity, opisthotonos, dyspnea and cardiac arrest. In the cat, myoclonic jerking and/or mild tonic convulsions can be controlled by ultrashort-acting barbiturates which should be given to effect. The barbiturates should be administered intravenously at a dose level of one-sixth to one-fourth the usual dose for the product being used. Acepromazine may also be used. However, recent information indicates that some phenothiazine derivatives may potentiate the toxic effects of organic phosphate compounds such as found in flea collars and certain anthelmintics. A study has indicated that ketamine hydrochloride alone does not potentiate the toxic effects of organic phosphate compounds.
To report suspected adverse reactions, to obtain a Material Safety Data Sheet or for technical assistance, call 1-866-683-0660.
Action:
Ketamine Hydrochloride Injection, USP is a rapid-acting agent whose pharmacological action is characterized by profound analgesia, normal pharyngeal-laryngeal reflexes, mild cardiac stimulation and respiratory depression. Skeletal muscle tone is variable and may be normal, enhanced or diminished. The anesthetic state produced does not fit into the conventional classification of stages of anesthesia, but instead Ketamine Hydrochloride Injection, USP produces a state of unconsciousness which has been termed “dissociative” anesthesia in that it appears to selectively interrupt association pathways to the brain before producing somesthetic sensory blockade.
In contrast to other anesthetics, protective reflexes, such as coughing and swallowing are maintained under Ketamine Hydrochloride Injection, USP anesthesia. The degree of muscle tone is dependent upon level of dose; therefore, variations in body temperature may occur. At low dosage levels there may be an increase in muscle tone and a concomitant slight increase in body temperature. However, at high dosage levels there is some diminution in muscle tone and a resultant decrease in body temperature, to the point where supplemental heat may be advisable.
In cats, there is usually some transient cardiovascular stimulation, increased cardiac output with slight increase in mean systolic pressure with little or no change in total peripheral resistance. At higher doses the respiratory rate is usually decreased.
The assurance of a patent airway is greatly enhanced by virtue of maintained pharyngeal-laryngeal reflexes. Although some salivation is occasionally noted, the persistence of the swallowing reflex aids in minimizing the hazards associated with ptyalism. Salivation may be effectively controlled with atropine sulfate in dosages of 0.04 mg/kg (0.02 mg/lb) in cats and 0.01 to 0.05 mg/kg (0.005 to 0.025 mg/lb) in subhuman primates.
Other reflexes, e.g., corneal, pedal, etc., are maintained during Ketamine Hydrochloride Injection, USP anesthesia, and should not be used as criteria for judging depth of anesthesia. The eyes normally remain open with the pupils dilated. It is suggested that a bland ophthalmic ointment be applied to the cornea if anesthesia is to be prolonged.
Following administration of recommended doses, cats become ataxic in about 5 minutes with anesthesia usually lasting from 30 to 45 minutes at higher doses. At the lower doses, complete recovery usually occurs in 4 to 5 hours but with higher doses recovery time is more prolonged and may be as long as 24 hours.
In studies involving 14 species of subhuman primates represented by at least 10 anesthetic episodes for each species, the median time to restraint ranged from 1.5 [Aotus trivirgatus (night monkey) and Cebus capucinus (white-throated capuchin)] to 5.3 minutes [Macaca nemestrina (pig-tailed macaque)]. The median duration of restraint ranged between 20 and 55 minutes in all but five of the species studied. Total time from injection to end of restraint ranged from 43 [Saimiri sciureus (squirrel monkey)] to 183 minutes [Macaca nemestrina (pig-tailed macaque)] after injection. Recovery is generally smooth and uneventful. The duration is dose related.
By single intramuscular injection, Ketamine Hydrochloride Injection, USP usually has a wide margin of safety in cats and subhuman primates. In cats, cases of prolonged recovery and death have been reported.
Clinical Studies:
Ketamine Hydrochloride Injection, USP has been clinically studied in subhuman primates in addition to those species listed under Dosage and Administration. Dosages for restraint in these additional species, based on limited clinical data, are: Cercopithecus aethiops (grivet), Papio papio (guinea baboon) 10 to 12 mg/kg; Erythrocebus patas patas (patas monkey) 3 to 5 mg/kg; Hylobates lar (white-handed gibbon) 5 to 10 mg/kg; Lemur catta (ringtailed lemur) 7.5 to 10 mg/kg; Macaca fuscata (Japanese macaque) 5 mg/kg; Macaca speciosa (stumptailed macaque) and Miopithecus talapoin (mangrove monkey) 5 to 7.5 mg/kg; and Symphalangus syndactylus (siamangs) 5 to 7 mg/kg.
Storage Information:
Store at or below 25°C (77°F). Protect from light. Use contents within 6 months of first puncture.
How Supplied:
Ketamine Hydrochloride Injection, USP is supplied as the hydrochloride in concentrations equivalent to ketamine base. Each 10 mL vial contains 100 mg/mL.
NDC: 26637-731-51 - 10 mL vial
| KETAMINE HYDROCHLORIDE
ketamine hydrochloride injection |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Putney, Inc. (792295243) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.