PRIORIX- measels, mumps, and rubella vaccine, live kit
Priorix by
Drug Labeling and Warnings
Priorix by is a Other medication manufactured, distributed, or labeled by GlaxoSmithKline Biologicals SA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PRIORIX safely and effectively. See full prescribing information for PRIORIX.
PRIORIX (Measles, Mumps, and Rubella Vaccine, Live) for injectable suspension, for subcutaneous use
Initial U.S. Approval: 2022RECENT MAJOR CHANGES
Contraindications, Immunosuppression (4.2)
11/2025
INDICATIONS AND USAGE
PRIORIX is a vaccine indicated for active immunization for the prevention of measles, mumps, and rubella in individuals 12 months of age and older. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
For injectable suspension. PRIORIX is supplied as a single-dose vial of Lyophilized Antigen Component, Live to be reconstituted with the accompanying prefilled syringe of Sterile Water Diluent Component. A single dose after reconstitution is approximately 0.5 mL. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- There is a risk of febrile seizure following administration of PRIORIX. (5.2)
- Thrombocytopenia and thrombocytopenic purpura have been reported following vaccination with PRIORIX. (5.3)
- Syncope (fainting) can occur in association with administration of injectable vaccines, including PRIORIX. Procedures should be in place to avoid injury from fainting. (5.4)
- The tip caps of the prefilled syringes contain natural rubber latex, which may cause allergic reactions. (5.5)
ADVERSE REACTIONS
Most common solicited adverse reactions in clinical trials participants:
- 12 through 15 months of age: local reactions were pain (26%) and redness (25%); systemic reactions were irritability (63%), loss of appetite (45%), drowsiness (45%), and fever (35%). (6.1)
- 4 through 6 years of age: local reactions were pain (41%), redness (22%), and swelling (11%); systemic reactions were loss of appetite (21%), drowsiness (27%), and fever (24%). (6.1)
- 7 years of age and older: local reactions were pain (12%) and redness (12%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Schedule
2.2 Preparation
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Severe Allergic Reactions
4.2 Immunosuppression
4.3 Pregnancy
5 WARNINGS AND PRECAUTIONS
5.1 Allergic Vaccine Reactions
5.2 Febrile Seizures
5.3 Thrombocytopenia
5.4 Syncope
5.5 Latex
5.6 Risk of Vaccine Virus Transmission
5.7 Limitation of Vaccine Effectiveness
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Immunosuppressive Drugs
7.2 Immune Globulins and Blood Products
7.3 Tuberculin Skin Testing
7.4 Use with Other Live Viral Vaccines
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Antibody Responses to Measles, Mumps and Rubella Viruses
14.2 Concomitant Administration
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 Storage before Reconstitution
16.2 Storage after Reconstitution
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For subcutaneous use.
2.1 Dose and Schedule
After reconstitution, a single dose of PRIORIX is approximately 0.5 mL.
Administer according to the following schedule:
- First dose – 12 through 15 months of age
- Second dose – 4 through 6 years of age
If PRIORIX is not administered according to this schedule and 2 doses of measles-, mumps- and rubella-virus vaccine are recommended for an individual, there should be a minimum of 4 weeks between the first and second dose.
PRIORIX may be administered as a second dose to individuals who have received a first dose of another measles, mumps and rubella virus-containing vaccine.
2.2 Preparation
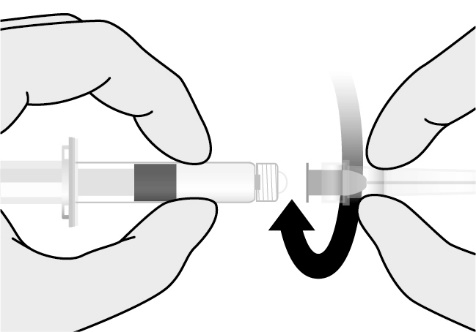
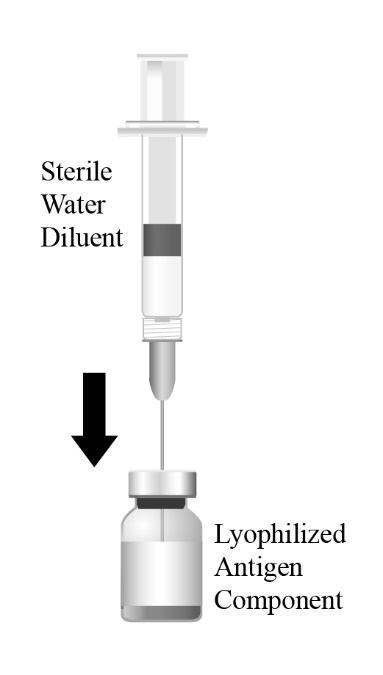
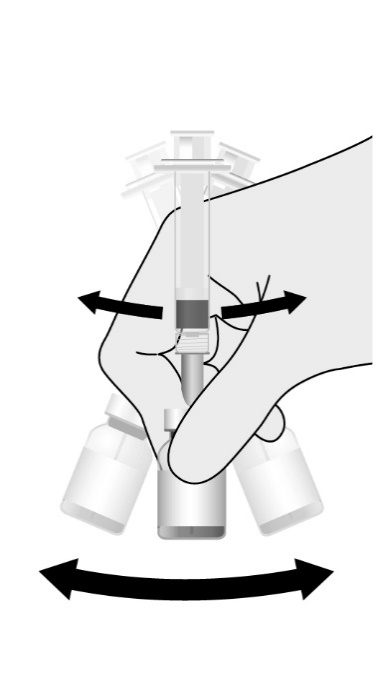
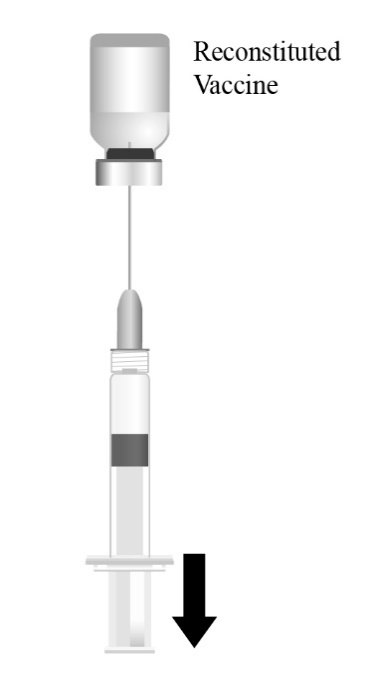
Reconstitute the Lyophilized Antigen Component, Live only with the accompanying Sterile Water Diluent Component to form PRIORIX. The reconstituted vaccine should be a clear peach- to fuchsia pink-colored suspension. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, do not administer the vaccine.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Severe Allergic Reactions
Do not administer PRIORIX to individuals with a history of severe allergic reactions (e.g., anaphylaxis) to any component of the vaccine or after a previous dose of any measles, mumps, and rubella virus-containing vaccine [see Description (11)].
4.2 Immunosuppression
Do not administer PRIORIX to individuals who are immunodeficient or immunosuppressed due to disease or medical therapy. These individuals are at risk of disseminated vaccine virus infection [see Drug Interactions (7.1)].
4.3 Pregnancy
Do not administer PRIORIX to individuals who are pregnant. Pregnancy should be avoided for 1 month after vaccination [see Use in Specific Populations (8.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Allergic Vaccine Reactions
Appropriate medical treatment used to manage immediate allergic reactions must be available in the event an acute anaphylactic reaction occurs following administration of PRIORIX.
5.2 Febrile Seizures
There is a risk of febrile seizure following immunization with PRIORIX [see Adverse Reactions (6.1)].
5.3 Thrombocytopenia
Thrombocytopenia and thrombocytopenic purpura have been reported following vaccination with PRIORIX [see Adverse Reactions (6.2)].
5.4 Syncope
Syncope (fainting) can occur in association with administration of injectable vaccines, including PRIORIX. Procedures should be in place to avoid injury from fainting.
5.5 Latex
The tip caps of the prefilled syringes of diluent contain natural rubber latex, which may cause allergic reactions.
-
6 ADVERSE REACTIONS
The most commonly reported (≥10%) solicited adverse reactions in the following age groups in clinical trials were:
- Age 12 through 15 months – local: pain (26%) and redness (25%); systemic: irritability (63%), loss of appetite (45%), drowsiness (45%), and fever (35%)
- Age 4 through 6 years – local: pain (41%), redness (22%), and swelling (11%); systemic: loss of appetite (21%), drowsiness (27%), and fever (24%)
- Age 7 years and older – local: pain (12%) and redness (12%)
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared with rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The safety of PRIORIX was evaluated in 6 clinical studies, in which a total of 12,151 participants (6,391 in the United States) received at least 1 dose of PRIORIX: 8,780 children (4,148 in the United States) 12 through 15 months of age; 2,917 children (1,950 in the United States) 4 through 6 years of age; and 454 adults and children (293 in the United States) 7 years of age and older. Across the 6 studies, participants who received PRIORIX are as follows: 51.6% were male; 64.6% were White, 18.4% were Asian, 6.1% were Black, and 10.9% were of other racial groups (including American Indian/Native American, Native Hawaiian/Pacific Islander, Arabic/North African and Other); and 14.3% were of Hispanic/Latino ethnicity. The racial/ethnic distribution of participants who received PRIORIX and M‑M‑R II was similar.
Children 12 through 15 Months of Age Who Received PRIORIX as a First Dose
In a randomized, observer-blind, controlled clinical study (Study 1, NCT01702428) conducted in 5 countries (United States [including Puerto Rico], Estonia, Finland, Mexico and Spain), 5,003 participants 12 through 15 months of age received a first dose of PRIORIX (n = 3,714) or M‑M‑R II (n = 1,289) given concomitantly with HAVRIX (Hepatitis A Vaccine) and VARIVAX (Varicella Virus Vaccine Live, Merck & Co., Inc.); children enrolled in the United States also received PREVNAR 13 (Pneumococcal 13-valent Conjugate Vaccine, Pfizer Inc.) concomitantly. In the overall population, 51.3% were male; 75.6% were White, 4.8% were Black, 3.5% were Asian, 16.1% were of other racial groups (including American Indian/Native American, Native Hawaiian/Pacific Islander, Arabic/North African and Other); and 18.6% were of Hispanic/Latino ethnicity. The median age of participants was 12 months (range: 11 to 16 months). Local solicited adverse reactions were recorded by parents or guardians using standardized diary cards for 4 days. Systemic solicited adverse reactions of drowsiness, loss of appetite, and irritability were collected for 15 days, and fever, rash, parotid/salivary gland swelling, febrile convulsions, and signs of meningeal irritation (i.e., neck stiffness with or without photophobia or headache) were collected for 43 days (Table 1). Unsolicited adverse events that occurred within 43 days following vaccination were recorded using diary cards supplemented by medical review. Data on solicited adverse reactions and unsolicited adverse events were transcribed into the study database during an on-site visit on Day 42 and via telephone contact on Day 180.
Table 1. Incidence of Solicited Local and Systemic Adverse Reactions after the First Dose of PRIORIX Compared with M-M-R II in Children 12 through 15 Months of Age (Study 1, NCT01702428, Total Vaccinated Cohort)a Total vaccinated cohort for safety included all vaccinated participants for whom safety data were available. N = Number of participants. n = Number of participants presenting with solicited adverse reaction described. a HAVRIX and VARIVAX were administered concomitantly with PRIORIX or M-M-R II; participants in the U.S. also received PREVNAR 13 concomitantly with PRIORIX (n = 1,847) or M-M-R II (n = 654). b 4 Days, 15 Days, and 43 Days included the day of vaccination and the subsequent 3, 14, and 42 days, respectively. c Neck stiffness with or without photophobia or headache. Adverse Reaction
PRIORIX
n (%)
M-M-R II
n (%)
Local (within 4 Daysb)
N = 3,555
N = 1,242
Pain
919 (25.9%)
349 (28.1%)
Redness
870 (24.5%)
313 (25.2%)
Swelling
318 (8.9%)
133 (10.7%)
Systemic (within 15 Daysb)
N = 3,566
N = 1,243
Drowsiness
1601 (44.9%)
586 (47.1%)
Irritability
2258 (63.3%)
819 (65.9%)
Loss of appetite
1608 (45.1%)
548 (44.1%)
Systemic (within 43 Daysb)
N = 3,566
N = 1,243
Measles/rubella-like rash
235 (6.6%)
77 (6.2%)
Fever (defined as temperature ≥38°C/100.4°F)
1244 (34.9%)
412 (33.1%)
Parotid/salivary gland swelling
0
0
Febrile convulsions
7 (0.2%)
3 (0.2%)
Signs of meningeal irritationc
3 (0.1%)
0
Children 12 through 15 Months of Age Who Received a Second Dose of PRIORIX 6 Weeks after the First Dose
In a randomized, observer-blind, controlled clinical study (Study 2, NCT01681992) conducted in six countries (United States [including Puerto Rico], Czech Republic, Finland, Malaysia, Spain and Thailand), 4,516 participants 12 through 15 months of age received a first dose of PRIORIX (n = 2,990) or M‑M‑R II (n = 1,526) followed by a second dose of the same vaccine 6 weeks later. The first dose was given concomitantly with HAVRIX and VARIVAX; children enrolled in the United States (including Puerto Rico) also received PREVNAR 13 concomitantly. In the overall population, 51.7% were male; 68.4% were White, 24.4% were Asian, 3.2% were Black, and 4.0% were of other racial groups (including American Indian/Native American, Native Hawaiian/Pacific Islander, Arabic/North African and Other); and 5.6% were of Hispanic/Latino ethnicity. The median age of participants was 12 months (range: 11 to 16 months). Local solicited adverse reactions were recorded by parents or guardians using standardized diary cards for 4 days, and systemic adverse reactions of fever, rash, parotid/salivary gland swelling, febrile convulsions, and signs of meningeal irritation (i.e., neck stiffness with or without photophobia or headache) were collected for 43 days. Unsolicited adverse events that occurred within 43 days following vaccination were recorded using diary cards supplemented by medical review. Data on solicited adverse reactions and unsolicited adverse events were transcribed into the study database during on-site visits on Day 42, Day 84, and Day 222. The safety profile of PRIORIX following the second dose was similar to the safety profile following the first dose of PRIORIX.
Children 4 through 6 Years of Age Who Received PRIORIX as a Second Dose of Measles, Mumps, and Rubella Vaccine
In a randomized, observer-blind, controlled clinical study (Study 3, NCT01621802) conducted in 3 countries (United States, South Korea, and Taiwan), 4,007 participants 4 through 6 years of age received PRIORIX (n = 2,917) or M-M-R II (n = 1,090) as a second dose following administration of an initial dose of a combined measles, mumps, and rubella virus-containing vaccine in the second year of life. PRIORIX and M-M-R II were given concomitantly with KINRIX (DTaP-IPV) [Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed and Inactivated Poliovirus Vaccine] and VARIVAX in a subset of subjects (n = 802 receiving PRIORIX, n = 298 receiving M-M-R II) enrolled in the United States. In the overall population, 52.5% were male; 42.4% were White, 37.2% were Asian, 8.2% were Black, and 12.3% were of other racial groups (including American Indian/Native American, Native Hawaiian/Pacific Islander, Arabic/North African and Other) and 17.2% were of Hispanic/Latino ethnicity. The median age of participants was 4 years (range: 3 to 6 years). In a subset of participants who received concomitantly administered vaccines, data on local solicited adverse reactions were recorded by parents or guardians using standardized diary cards for 4 days. Systemic solicited adverse reactions of drowsiness and loss of appetite were collected for 4 days, and fever, rash, parotid/salivary gland swelling, febrile convulsions, and signs of meningeal irritation (i.e., neck stiffness with or without photophobia or headache) were collected for 43 days (Table 2). Unsolicited adverse events that occurred within 43 days following vaccination were recorded using diary cards supplemented by medical review. Data on solicited adverse reactions and unsolicited adverse events were transcribed into the study database during an on‑site visit on Day 42 and via telephone contact on Day 180.
Table 2. Incidence of Solicited Local and Systemic Adverse Reactions after the Second Dose of PRIORIX Compared with M-M-R II Concomitantly Administered with KINRIX and VARIVAX in Children 4 through 6 Years of Age (Study 3, NCT01621802, Total Vaccinated Cohort) Total vaccinated cohort for safety included all vaccinated participants for whom safety data were available. N = Number of participants. n = Number of participants presenting with solicited adverse reaction described. a 4 Days and 43 Days included the day of vaccination and the subsequent 3 and 42 days, respectively. b Neck stiffness with or without photophobia or headache. Adverse Reaction
PRIORIX
n (%)
M-M-R II
n (%)
Local (within 4 Daysa)
N = 727
N = 267
Pain
295 (40.6%)
109 (40.8%)
Redness
157 (21.6%)
69 (25.8%)
Swelling
82 (11.3%)
28 (10.5%)
Systemic (within 4 Daysa)
N = 731
N = 268
Drowsiness
199 (27.2%)
72 (26.9%)
Loss of appetite
154 (21.1%)
59 (22.0%)
Systemic (within 43 Daysa)
N = 731
N = 268
Measles/rubella-like rash
14 (1.9%)
5 (1.9%)
Fever (defined as temperature ≥38°C/100.4°F)
177 (24.2%)
67 (25.0%)
Parotid/salivary gland swelling
0
0
Febrile convulsions
0
0
Signs of meningeal irritationb
0
2 (0.7%)
Individuals 7 Years of Age and Older Who Received PRIORIX as a Second Dose of Measles, Mumps, and Rubella Vaccine
In a randomized, observer-blind, controlled clinical study (Study 4, NCT02058563) conducted in 3 countries (United States, Slovakia, and Estonia), 860 participants 7 years of age and older received PRIORIX (n = 426) or M-M-R II (n = 434) as a second dose following previous administration of a combined measles, mumps, and rubella virus-containing vaccine. Participants 7 through 17 years were enrolled if they had received one dose of a combined measles, mumps, and rubella virus-containing vaccine on or after their first birthday and participants 18 years of age or older were enrolled if they previously received at least one dose of a combined measles, mumps, and rubella virus-containing vaccine. In the overall population, 46.2% were male; 73.8% were White, 0.2% were Asian, 24.0% were Black, and 1.9% were of other racial groups (including American Indian/Native American, Native Hawaiian/Pacific Islander, Arabic/North African and Other) and 13.3% were of Hispanic/Latino ethnicity. The median age of participants was 26 years (range: 7 to 59 years). Data on solicited local and systemic adverse reactions were recorded by the participants or their parents or guardians using standardized diary cards for 4 days and 43 days, respectively (Table 3). Unsolicited adverse events that occurred within 43 days following vaccination were recorded using diary cards supplemented by medical review. Data on solicited adverse reactions and unsolicited adverse events were transcribed into the study database during an on-site visit on Day 42 and via telephone contact on Day 180.
Table 3. Incidence of Solicited Local and Systemic Adverse Reactions after PRIORIX as a Second Dose Compared with M-M-R II in Individuals 7 Years of Age and Older (Study 4, NCT02058563, Total Vaccinated Cohort)a Total vaccinated cohort for safety included all vaccinated participants for whom safety data were available. N = Number of participants. n = Number of participants presenting with solicited adverse reaction described. a Participants received a first dose of either M-M-R II, PRIORIX, or a non-U.S. combined measles, mumps, rubella and varicella virus vaccine. b 4 Days and 43 Days included the day of vaccination and the subsequent 3 and 42 days, respectively. c Neck stiffness with or without photophobia or headache. PRIORIX
n (%)
M-M-R II
n (%)
Local (within 4 Daysb)
N = 405
N = 422
Pain
49 (12.1%)
47 (11.1%)
Redness
48 (11.9%)
50 (11.8%)
Swelling
23 (5.7%)
29 (6.9%)
Systemic (within 43 Daysb)
N = 405
N = 422
Fever (defined as temperature ≥38°C/100.4°F)
11 (2.7%)
23 (5.5%)
Measles/rubella-like rash
0
2 (0.5%)
Joint pain (arthralgia/arthritis)
8 (2.0%)
4 (0.9%)
Parotid/salivary gland swelling
1 (0.2%)
0
Signs of meningeal irritationc
1 (0.2%)
1 (0.2%)
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been identified during postmarketing use of PRIORIX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccination with PRIORIX.
Blood and Lymphatic System Disorders
Thrombocytopenia, thrombocytopenic purpura.
Vascular Disorders
Vasculitis (including Henoch-Schönlein purpura and Kawasaki syndrome).
Immune System Disorders
Anaphylactic reactions.
Infections and Infestations
Meningitis, measles-like illness, mumps-like illness (including orchitis, epididymitis, and parotitis).
Musculoskeletal and Connective Tissue Disorders
Arthralgia, arthritis.
Nervous System Disorders
Encephalitis, cerebellitis, cerebellitis-like symptoms (including transient gait disturbance and transient ataxia), Guillain-Barré syndrome, transverse myelitis, peripheral neuritis, afebrile seizures, syncope.
Skin and Subcutaneous Tissue Disorders
Erythema multiforme, chronic cutaneous granulomas with rubella vaccine virus detected by biopsy.
-
7 DRUG INTERACTIONS
7.1 Immunosuppressive Drugs
Do not administer PRIORIX to individuals who are immunosuppressed due to medical therapy. Vaccination with PRIORIX can result in disseminated disease due to vaccine viruses in individuals on immunosuppressive drugs [see Contraindications (4.2)].
7.2 Immune Globulins and Blood Products
Immune globulins and other blood products administered concomitantly with PRIORIX contain antibodies that may interfere with vaccine virus replication and decrease the expected immune response. The U.S. Centers for Disease Control and Prevention (CDC) has specific recommendations for intervals between administration of antibody containing products and live virus vaccines.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
PRIORIX contains live attenuated measles, mumps, and rubella viruses. The vaccine is contraindicated for use in pregnant women because infection during pregnancy with the wild-type viruses is associated with maternal and fetal adverse outcomes. Pregnancy should be avoided for 1 month after vaccination [see Contraindications (4.3), Patient Counseling Information (17)].
Reports have indicated that contracting wild-type measles during pregnancy enhances fetal risk, including increased rates of spontaneous abortion, stillbirth, premature delivery and congenital defects.2,3 Wild-type mumps virus infection during the first trimester of pregnancy may increase the rate of spontaneous abortion. Pregnant women infected with wild-type rubella virus are at increased risk for miscarriage or stillbirth, and their infants are at risk for congenital rubella syndrome (CRS).1
Postmarketing surveillance has identified a case of CRS following inadvertent vaccination of a pregnant woman with a measles, mumps, and rubella virus containing vaccine from an unknown manufacturer4[see Data].
There are no animal studies with PRIORIX to inform use during pregnancy.
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data: Postmarketing surveillance has identified a case of CRS associated with a rubella virus strain belonging to the genotype that includes the rubella virus strain Wistar RA 27/3 contained in PRIORIX. The infant with CRS was born to a pregnant woman who was inadvertently vaccinated at 5 weeks gestation with a measles, mumps, and rubella virus containing vaccine from an unknown manufacturer.4
8.2 Lactation
Risk Summary
It is not known whether the vaccine components of PRIORIX are excreted in human milk. Data are not available to assess the effects of PRIORIX on the breastfed infant or on milk production/excretion. Studies have shown that lactating postpartum women vaccinated with live attenuated rubella vaccine may secrete the virus in breast milk and transmit it to breast‑fed infants.5,6 In the breast-fed infants with serological evidence of rubella virus vaccine strain antibodies, none exhibited severe disease; however, one exhibited mild clinical illness typical of acquired rubella.7,8
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for PRIORIX and any potential adverse effects on the breastfed child from PRIORIX or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
-
11 DESCRIPTION
PRIORIX (Measles, Mumps, and Rubella Vaccine, Live) is an injectable suspension for subcutaneous use. PRIORIX is supplied as a sterile, Lyophilized Antigen Component, Live which is reconstituted at the time of use with the accompanying Sterile Water Diluent Component. The Lyophilized Antigen Component, Live is a whitish to slightly pink powder, a portion of which may be yellowish to slightly orange.
PRIORIX contains the Schwarz strain of live attenuated measles virus, the RIT 4385 strain of live attenuated mumps virus (derived from the Jeryl Lynn strain), both propagated in chick‑embryo fibroblasts from embryonated eggs of specific pathogen-free flocks and the Wistar RA 27/3 strain of live attenuated rubella virus propagated in MRC-5 human diploid cells. The 3 virus strains are cultured in media containing amino acids, a small amount of neomycin sulfate and bovine serum albumin and are stabilized after multiple washing steps in media free from antibiotics and albumin. The attenuated measles, mumps and rubella viruses are then mixed with a stabilizer prior to lyophilization.
After reconstitution, each approximately 0.5-mL dose contains not less than 3.4 log10 Cell Culture Infective Dose 50% (CCID50) of measles virus, 4.2 log10 CCID50 of mumps virus, and 3.3 log10 CCID50 of rubella virus. Each dose also contains 32 mg of anhydrous lactose, 9 mg of sorbitol, 9 mg of amino acids, and 8 mg of mannitol. Each dose may also contain residual amounts of neomycin sulphate (≤25 mcg), ovalbumin (≤60 ng), and bovine serum albumin (≤50 ng), from the manufacturing process. After reconstitution, PRIORIX is a clear peach- to fuchsia pink-colored suspension.
PRIORIX contains no preservative.
The tip caps of the prefilled syringes of Sterile Water Diluent Component contain natural rubber latex. The plungers of the syringes and the stoppers of the Lyophilized Antigen Component, Live vials are not made with natural rubber latex.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Humoral immune responses against measles, mumps, and rubella viruses induced by PRIORIX were measured by enzyme-linked immunosorbent assays (ELISAs). IgG antibodies measured by the ELISAs used in clinical studies of PRIORIX have been shown to correlate with the presence of neutralizing antibodies that have been associated with protection [see Clinical Studies (14)].
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The effectiveness of PRIORIX is based on a comparison of antibody responses relative to M‑M‑R II. Antibody responses to measles, mumps, and rubella viruses were measured by ELISAs. Analyses evaluated antibody geometric mean concentrations (GMC) and seroresponse rates (SRR). Seroresponse thresholds are 200 mIU/mL, 10 ELU/mL, and 10 IU/mL for anti-measles virus, anti-mumps virus, and anti-rubella virus antibodies, respectively.
14.1 Antibody Responses to Measles, Mumps and Rubella Viruses
Children 12 through 15 Months of Age Who Received PRIORIX as a First Dose
In Study 1 (NCT01702428), 5,003 participants 12 through 15 months of age received a first dose of PRIORIX (n = 3,714) or M‑M‑R II (n = 1,289) [see Adverse Reactions (6.1)]. Antibody responses to measles, mumps, and rubella viruses were measured by ELISAs using sera obtained 42 days following the first dose of either PRIORIX or M-M-R II. Non-inferiority of the immune response after the first dose of PRIORIX compared with M-M-R II was demonstrated in terms of SRR and GMC to measles, mumps, and rubella viruses. The immune responses measured in the U.S. study participants were similar to those in the overall study population. A summary of immune responses is shown in Table 4.
Table 4. Immune Responses after the First Dose of PRIORIX Compared with M-M-R II (Study 1, NCT01702428, According-to-Protocol Population) According-to-Protocol cohort included all vaccinated participants who met protocol-defined criteria for immunogenicity analysis. PRIORIX or M-M-R II was administered concomitantly with HAVRIX and VARIVAX; U.S. participants also received PREVNAR 13. N = Number of participants. SRR = Seroresponse rate (percentage of initially seronegative participants with concentration above seroresponse threshold for each assay). GMC = Geometric mean antibody concentration adjusted for country. CI = Confidence Interval. a Non-inferiority criterion met for all antigens (lower limit of 2-sided 95% CI for the difference [group receiving PRIORIX minus group receiving M-M-R II] was ≥-5%). b Non-inferiority criterion met for all antigens (lower limit of 2-sided 95% CI for the ratio [group receiving PRIORIX over group receiving M-M-R II] was ≥0.67). Parameter
Virus Antigen
PRIORIX
N = 3,187-3,248
M-M-R II
N = 1,107-1,137
Difference
(PRIORIX minus
M-M-R II)
(95% CI)
SRRa (%)
Measles
98
98
0.18
(-0.68, 1.25)
Mumps
98
98
0.81
(-0.10, 1.96)
Rubella
97
99
-1.15
(-2.00, -0.15)
PRIORIX
N = 3,187-3,248
M-M-R II
N = 1,107-1,137
Ratio
(PRIORIX / M-M-R II)
(95% CI)
GMCb
Measles (mIU/mL)
3,165
3,215
0.98
(0.93, 1.05)
Mumps (ELU/mL)
76
73
1.05
(0.99, 1.11)
Rubella (IU/mL)
53
60
0.87
(0.83, 0.92)
Children 12 through 15 Months of Age Who Received a Second Dose of PRIORIX 6 Weeks after the First Dose
In Study 2 (NCT01681992), 4,516 participants 12 through 15 months of age received a first dose of PRIORIX (n = 2,990) or M-M-R II (n = 1,526) followed by a second dose of the same vaccine 6 weeks later [see Adverse Reactions (6.1)]. Antibody responses to measles, mumps, and rubella viruses were measured in a subset of participants (n = 199 – 259 PRIORIX; n = 212 – 257 M‑M‑R II) in sera obtained 42 days following the second dose of either PRIORIX or M‑M‑R II. In a descriptive analysis, the immune response after a second dose was similar between the group receiving PRIORIX and the group receiving M-M-R II in terms of antibody SRR and GMC for all antigens.
Children 4 through 6 Years of Age Who Received PRIORIX as a Second Dose of Measles, Mumps, and Rubella Virus Vaccine
In Study 3 (NCT01621802), 4,007 participants 4 through 6 years of age received PRIORIX (n = 2,917) or M‑M‑R II (n = 1,090) as a second dose following administration of an initial dose of a combined measles, mumps, and rubella virus-containing vaccine in the second year of life [see Adverse Reactions (6.1)]. Prior to vaccination, the percentages of participants with antibody levels above the seroresponse thresholds were 98.0% for measles, 95.7% for mumps, and 98.7% for rubella. Antibody responses to measles, mumps, and rubella viruses were measured by ELISAs using sera obtained 42 days following of either PRIORIX or M‑M‑R II as a second dose. The non-inferiority of PRIORIX to M-M-R II when administered with KINRIX and VARIVAX was demonstrated in terms of SRR and GMC to measles, mumps, and rubella viruses at Day 42 (Table 5).
Table 5. Immune Responses to PRIORIX Compared with M-M-R II as a Second Dose in Children 4 through 6 Years of Age (Study 3, NCT01621802, According-to-Protocol Population) According-to-Protocol cohort included all vaccinated participants who met protocol-defined criteria for immunogenicity analysis. N = Number of participants. SRR = Seroresponse rate (percentage of participants with concentration above seroresponse threshold for each assay). GMC = Geometric mean antibody concentration adjusted for pre-vaccination concentration. CI = Confidence Interval. a Non-inferiority criterion met for all antigens (lower limit of 2-sided 97.5% CI for the difference [group receiving PRIORIX minus group receiving M-M-R II] was ≥-5%). b Non-inferiority criterion met for all antigens (lower limit of 2-sided 97.5% CI for the ratio [group receiving PRIORIX over group receiving M-M-R II] was ≥0.67). Parameter
Virus Antigen
PRIORIX
N = 690-698
M-M-R II
N = 245-250
Difference
(PRIORIX minus
M-M-R II)
(97.5% CI)
SRRa (%)
Measles
100
100
0.00
(-0.72, 1.98)
Mumps
100
100
0.00
(-0.72, 1.97)
Rubella
100
100
-0.14
(-0.98, 1.84)
PRIORIX
N = 690-691
M-M-R-II
N = 245-248
Ratio
(PRIORIX / M-M-R II)
(97.5% CI)
GMCb
Measles (mIU/mL)
4,285
4,333
0.99
(0.92, 1.06)
Mumps (ELU/mL)
171
188
0.91
(0.83, 1.00)
Rubella (IU/mL)
97
94
1.03
(0.97, 1.09)
Individuals 7 Years of Age and Older Who Received PRIORIX as a Second Dose of Measles, Mumps, and Rubella Vaccine
In Study 4 (NCT02058563), 860 participants 7 years of age and older received PRIORIX (n = 426) or M‑M‑R II (n = 434) as a second dose following previous administration of a combined measles, mumps, and rubella virus-containing vaccine [see Adverse Reactions (6.1)]. Prior to vaccination, the percentages of participants with antibody levels above the seroresponse thresholds were 93.1% for measles, 88.0% for mumps, and 81.9% for rubella. Antibody responses to measles, mumps, and rubella viruses were measured in sera obtained 42 days following the second dose of either PRIORIX or M-M-R II. The non-inferiority of the immune response after the second dose of PRIORIX compared with M-M-R II was demonstrated in terms of SRR and antibody GMC to measles, mumps, and rubella antigens. A summary of immune responses is shown in Table 6.
Table 6. Immune Responses to PRIORIX as a Second Dose Compared with M-M-R II (Study 4, NCT02058563, According-to-Protocol Population) According-to-Protocol cohort included all vaccinated participants who met protocol-defined criteria for immunogenicity analysis. N = Number of participants. SRR = Seroresponse rate (percentage of participants with concentration above seroresponse threshold for each assay). GMC = Geometric mean antibody concentration adjusted for gender, age, country, and pre‑vaccination concentration. CI = Confidence Intervals. a Non-inferiority criterion met for all antigens (lower limit of 2-sided 95% CI for the difference [group receiving PRIORIX minus group receiving M-M-R II] was ≥-5%). b Non-inferiority criterion met for all antigens (lower limit of 2-sided 95% CI for the ratio [group receiving PRIORIX over group receiving M-M-R II] was ≥0.67). Parameter
Virus Antigen
PRIORIX
N = 405
M-M-R II
N = 414
Difference
(PRIORIX minus
M-M-R II)
(95% CI)
SRRa (%)
Measles
99
99
-0.51
(-2.22, 1.02)
Mumps
98
100
-1.25
(-3.10, 0.23)
Rubella
100
100
-0.25
(-1.57, 0.90)
PRIORIX
N = 404
M-M-R II
N = 413
Ratio
(PRIORIX / M‑M‑R II) (95% CI)
GMCb
Measles (mIU/mL)
1,754
1,783
0.98
(0.89, 1.09)
Mumps (ELU/mL)
114
110
1.04
(0.94, 1.15)
Rubella (IU/mL)
76
74
1.03
(0.94, 1.12)
14.2 Concomitant Administration
Concomitant Administration with HAVRIX, VARIVAX, and PREVNAR 13
The concomitant use of PRIORIX or M-M-R II with HAVRIX and VARIVAX was evaluated in Study 1 (NCT01702428) in children 12 through 15 months of age. All participants received PRIORIX or M-M-R II administered concomitantly with HAVRIX and VARIVAX. Children enrolled in the U.S. also received PREVNAR 13 concomitantly.
In subsets of participants in Study 1, immune responses to the antigens contained in HAVRIX, VARIVAX, and PREVNAR 13 were measured in sera obtained 42 days after concomitant administration of PRIORIX or M-M-R II. There was no evidence that PRIORIX interfered with the antibody responses to these vaccines relative to the antibody responses when M-M-R II was concomitantly administered.
Concomitant Administration with KINRIX and VARIVAX
The concomitant use of PRIORIX or M-M-R II with KINRIX and VARIVAX was evaluated in Study 3 (NCT01621802) in children 4 through 6 years of age. A subset of participants received PRIORIX or M-M-R II administered concomitantly with KINRIX and VARIVAX.
Immune responses to the antigens contained in KINRIX and VARIVAX were measured in sera obtained 42 days after concomitant administration of PRIORIX or M‑M‑R II. There was no evidence that PRIORIX interfered with the antibody responses to these vaccines relative to the antibody responses when M-M-R II was concomitantly administered.
-
15 REFERENCES
- 1. Centers for Disease Control and Prevention. Measles, Mumps, and Rubella- Vaccine Use and Strategies for Elimination of Measles, Rubella, and Congenital Rubella Syndrome and Control of Mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2013; 62(4);1-34.
- 2. Eberhart-Phillips, J.E.; et al: Measles in pregnancy: a descriptive study of 58 cases. Obstetrics and Gynecology, 82(5): 797-801, November 1993.
- 3. Jespersen, C.S.; et al: Measles as a cause of fetal defects: A retrospective study of ten measles epidemics in Greenland. Acta Paediatr Scand. 66: 367-372, May 1977.
- 4. Bouthry, E.; Queinnec, C.; Vauzelle, C.; Vauloup‑Fellous, C.: Congenital rubella syndrome following rubella vaccination during pregnancy. Pediatrics. 2023; 152 (3): e2022057627.
- 5. Losonsky, G.A.; Fishaut, J.M.; Strussenber, J.; Ogra, P.L.: Effect of immunization against rubella on lactation products. II. Maternal-neonatal interactions, J. Infect. Dis. 145: 661-666, 1982.
- 6. Losonsky, G.A.; Fishaut, J.M.; Strussenber, J.; Ogra, P.L.: Effect of immunization against rubella on lactation products. I. Development and characterization of specific immunologic reactivity in breast milk, J. Infect. Dis. 145: 654-660, 1982.
- 7. Landes, R.D.; Bass, J.W.; Millunchick, E.W.; Oetgen, W.J.: Neonatal rubella following postpartum maternal immunization, J. Pediatr. 97: 465-467, 1980.
- 8. Lerman, S.J.: Neonatal rubella following postpartum maternal immunization, J. Pediatr. 98: 668, 1981. (Letter)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
PRIORIX is supplied in a box (NDC: 58160-824-15) containing:
- 10 single-dose vials of Lyophilized Antigen Component, Live: NDC: 58160-831-03
- 10 single-dose prefilled ungraduated syringes of Sterile Water Diluent Component (packaged without needles): NDC: 58160-833-02
After reconstitution, each vial contains one dose (approximately 0.5 mL) of PRIORIX.
16.1 Storage before Reconstitution
Vials of Lyophilized Antigen Component, Live: Store refrigerated between 36° and 46°F (2° and 8°C). Protect vials from light.
Prefilled ungraduated syringes of Sterile Water Diluent Component: Store refrigerated between 36° and 46°F (2° and 8°C) or at controlled room temperature up to 77°F (25°C).
Do not freeze Lyophilized Antigen Component, Live or Sterile Water Diluent Component.
16.2 Storage after Reconstitution
Administer PRIORIX immediately after reconstitution. If not used immediately, store refrigerated between 36° and 46°F (2° and 8°C) and administer within 8 hours. Discard reconstituted vaccine if not used within 8 hours.
Do not freeze. Discard if the reconstituted vaccine has been frozen.
-
17 PATIENT COUNSELING INFORMATION
- Inform vaccine recipients, parents, or guardians of the potential benefits and risks of vaccination with PRIORIX.
- Question individuals of reproductive potential regarding the possibility of pregnancy prior to administration of PRIORIX. Instruct these individuals to avoid pregnancy for 1 month following vaccination [see Contraindications (4.3), Use in Specific Populations (8.1)].
- Inform vaccine recipients, parents, or guardians about the potential for adverse reactions that have been observed following administration of PRIORIX.
- Provide the Vaccine Information Statements, which are available free of charge at the U.S. Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
PRIORIX, HAVRIX, and KINRIX are trademarks owned by or licensed to the GSK group of companies.
The other brands listed are trademarks owned by or licensed to their respective owners and are not owned by or licensed to the GSK group of companies. The makers of these brands are not affiliated with and do not endorse the GSK group of companies or its products.
Manufactured by GlaxoSmithKline Biologicals
Rixensart, Belgium, U.S. License 1617
Distributed by GlaxoSmithKline, Durham, NC 27701
©2025 GSK group of companies or its licensor.
PRX:3PI
-
PRINCIPAL DISPLAY PANEL
NDC: 58160-824-15
PRIORIX
Measles, Mumps, and Rubella Vaccine, Live
MMR
Rx only
NOTICE: Reconstitue Lyophilized Antigen Component, Live with Sterile Water Diluent Component before use
For 12 Months of Age and Older
Contents: 10 Doses of PRIORIX
- 10 single-dose vials of Lyophilized Antigen Component, Live
- 10 single-dose prefilled ungraduated syringes of Sterile Water Diluent Component
After reconstitution, a single dose of PRIORIX is approximately 0.5 mL
- For subcutaneous injection only.
- GSK
- PRIORIX
- ©2024 the GSK group of companies or its licensor.
- Rev. 04/24
- 519679
-
INGREDIENTS AND APPEARANCE
PRIORIX
measels, mumps, and rubella vaccine, live kitProduct Information Product Type VACCINE Item Code (Source) NDC: 58160-824 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58160-824-15 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 VIAL 5 mL Part 2 10 SYRINGE 5 mL Part 1 of 2 PRIORIX
measels, mumps, and rubella vaccine, live injection, powder, lyophilized, for suspensionProduct Information Item Code (Source) NDC: 58160-831 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MEASLES VIRUS STRAIN SCHWARTZ ATTENUATED CHICK EMBRYO FIBROBLASTS LIVE ANTIGEN (UNII: YRF2UZG52M) (MEASLES VIRUS STRAIN SCHWARTZ ATTENUATED CHICK EMBRYO FIBROBLASTS LIVE ANTIGEN - UNII:YRF2UZG52M) MEASLES VIRUS STRAIN SCHWARTZ ATTENUATED CHICK EMBRYO FIBROBLASTS LIVE ANTIGEN 2512 [CCID_50] in 0.5 mL MUMPS VIRUS STRAIN RIT-4385 ATTENUATED CHICK EMBRYO FIBROBLASTS LIVE ANTIGEN (UNII: 566FJ5L8R4) (MUMPS VIRUS STRAIN RIT-4385 ATTENUATED CHICK EMBRYO FIBROBLASTS LIVE ANTIGEN - UNII:566FJ5L8R4) MUMPS VIRUS STRAIN RIT-4385 ATTENUATED CHICK EMBRYO FIBROBLASTS LIVE ANTIGEN 15842 [CCID_50] in 0.5 mL RUBELLA VIRUS STRAIN WISTAR RA 27/3 LIVE ANTIGEN (UNII: 52202H034Z) (RUBELLA VIRUS STRAIN WISTAR RA 27/3 LIVE ANTIGEN - UNII:52202H034Z) RUBELLA VIRUS STRAIN WISTAR RA 27/3 LIVE ANTIGEN 1995 [CCID_50] in 0.5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SORBITOL (UNII: 506T60A25R) MANNITOL (UNII: 3OWL53L36A) AMINO ACIDS, SOURCE UNSPECIFIED (UNII: 0O72R8RF8A) Product Characteristics Color WHITE (whitish to slightly pink) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58160-831-03 0.5 mL in 1 VIAL; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125748 06/03/2022 Part 2 of 2 DILUENT
water injection, solutionProduct Information Item Code (Source) NDC: 58160-833 Route of Administration SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58160-833-02 0.5 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125748 06/03/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125748 06/03/2022 Labeler - GlaxoSmithKline Biologicals SA (372748392)
Trademark Results [Priorix]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PRIORIX 97760716 not registered Live/Pending |
GlaxoSmithKline Biologicals SA 2023-01-19 |
 PRIORIX 97254008 not registered Live/Pending |
GlaxoSmithKline Biologicals SA 2022-02-04 |
 PRIORIX 85510276 4834274 Live/Registered |
GlaxoSmithKline Biologicals S.A. 2012-01-06 |
 PRIORIX 78584269 3063468 Dead/Cancelled |
GlaxoSmithKline Biologicals S.A. 2005-03-10 |
 PRIORIX 76425643 2785683 Dead/Cancelled |
SmithKline Beecham Biologicals, S.A. 2002-06-25 |
 PRIORIX 75457596 2232310 Dead/Cancelled |
SmithKline Beecham Biologicals S.A. 1998-03-26 |
 PRIORIX 75449199 not registered Dead/Abandoned |
SmithKline Beecham Biologicals S.A. 1998-03-12 |
 PRIORIX 75138244 not registered Dead/Abandoned |
SmithKline Beecham Biologicals S.A. 1996-07-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.