SPY-PHI KIT- indocyanine green kit SPY ELITE KIT- indocyanine green kit SPY LYMPHATICS KIT- indocyanine green kit PINPOINT LYMPHATICS KIT- indocyanine green kit SPY-MIS KIT- indocyanine green kit
PINPOINT Lymphatics Kit by
Drug Labeling and Warnings
PINPOINT Lymphatics Kit by is a Prescription medication manufactured, distributed, or labeled by Novadaq Technologies Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SPY AGENT™ GREEN safely and effectively. See full prescribing information for SPY AGENT™ GREEN.
SPY AGENT™ GREEN (indocyanine green for injection), intravenous or interstitial use.
Initial U.S. Approval: 1959INDICATIONS AND USAGE
SPY AGENT™ GREEN is an optical imaging agent indicated for use with a fluorescence imaging device for:
- Visualization of vessels (micro and macro vasculature), blood flow and tissue perfusion before, during, and after vascular, gastrointestinal, organ transplant, and plastic, micro- and reconstructive surgeries including general minimally invasive surgical procedures in adults and pediatric patients one month of age and older. ( 1.1)
- Visualization of extrahepatic biliary ducts in adults and pediatric patients aged 12 to 17 years. ( 1.2)
- Visualization of lymph nodes and lymphatic vessels during lymphatic mapping in women with cervical and uterine tumors. ( 1.3)
DOSAGE AND ADMINISTRATION
Visualization of Vessels, Blood Flow and Tissue Perfusion (2.5 mg/ml solution):
- The recommended single intravenous dose in adults and pediatric patients one month and older for a surgical procedure is 1.25 mg to 5 mg.
- The recommended single intravenous dose in adults for visualization of perfusion in extremities through the skin for plastic, micro, and reconstructive surgeries is 3.75 mg to 10 mg.
Additional doses may be administered to obtain additional imaging sequences during the procedure, however, do not exceed a total dose of 2 mg/kg. ( 2.1)
Visualization of Extrahepatic Biliary Ducts (2.5 mg/ml solution):
- The recommended single intravenous dose in adults and pediatric patients aged 12 to 17 years is 2.5 mg SPY AGENT GREEN.
- Inject SPY AGENT GREEN approximately 45 minutes prior to surgery to allow indocyanine green to collect in the biliary anatomy.
Additional doses may be administered to obtain additional imaging sequences during the procedure, however, do not exceed a total dose of 2 mg/kg. ( 2.2)
Visualization of Lymph Nodes and Lymphatic Vessels During Lymphatic Mapping for cervical and uterine tumors (1.25 mg/ml solution):
- The recommended single interstitial dose in women is four 1.25 mg injections for a total dose of 5 mg. Inject at the 3 and 9 o’clock positions of the cervix with a superficial (1 to 3 mm) and deep (1 to 3 cm) injection at each position. ( 2.3)
See Full Prescribing Information for instructions on reconstitution of the lyophilized powder, and preparation and administration of injections. ( 2.4)
DOSAGE FORMS AND STRENGTHS
For injection: SPY AGENT GREEN is a sterile lyophilized green powder containing 25 mg indocyanine green in a single-patient use vial for reconstitution. ( 3)
CONTRAINDICATIONS
History of hypersensitivity to indocyanine green. ( 4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions: Hypersensitivity reactions including anaphylaxis and urticaria have occurred with administration of indocyanine green. Always have cardiopulmonary resuscitation personnel and equipment readily available and monitor all patients for hypersensitivity reactions. ( 5.1)
- Interference with Radioactive Iodine Uptake Studies: Do not perform radioactive iodine uptake studies for at least a week following the use of SPY AGENT GREEN ( 5.2)
To report SUSPECTED ADVERSE REACTIONS, contact Stryker Corp. at 1-800-624-4422 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Revised: 2/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Visualization of Vessels, Blood Flow and Tissue Perfusion
1.2 Visualization of Extrahepatic Biliary Ducts
1.3 Visualization of Lymph Nodes and Lymphatic Vessels During Lymphatic Mapping for Cervical and Uterine Tumors
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose and Administration for Visualization of Vessels, Blood Flow and Tissue Perfusion
2.2 Recommended Dose and Administration for Visualization of Extrahepatic Biliary Ducts
2.3 Recommended Dose and Administration for Visualization of Lymph Nodes and Lymphatic Vessels During Lymphatic Mapping for Cervical and Uterine Tumors
2.4 Reconstitution Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Interference with Radioactive Iodine Uptake Studies
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Visualization of Vessels, Blood Flow and Tissue Perfusion
SPY AGENT™ GREEN is indicated in adults and pediatric patients one month of age and older for:
- fluorescence imaging of micro- and macro-vasculature, blood flow and tissue perfusion before, during and after vascular, gastrointestinal, organ transplant, and plastic, micro- and reconstructive surgeries, including general minimally invasive surgical procedures.
1.2 Visualization of Extrahepatic Biliary Ducts
SPY AGENT™ GREEN is indicated in adults and pediatric patients aged 12 to 17 years for:
- fluorescence imaging of extrahepatic biliary ducts.
1.3 Visualization of Lymph Nodes and Lymphatic Vessels During Lymphatic Mapping for Cervical and Uterine Tumors
SPY AGENT™ GREEN is indicated in women for:
- fluorescence imaging of lymph nodes and delineation of lymphatic vessels in the cervix and uterus during lymphatic mapping in patients with solid tumors for which this procedure is a component of intraoperative management.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose and Administration for Visualization of Vessels, Blood Flow and Tissue Perfusion
Dosing
Adults:
The recommended dose of SPY AGENT GREEN for a single image sequence is 1.25 mg to 5 mg of a 2.5 mg/mL solution.For visualization of perfusion in extremities through the skin, the recommended dose is 3.75 mg to 10 mg of a 2.5 mg/mL solution.
Immediately flush with a 10 mL bolus of 0.9% Sodium Chloride, USP.
Pediatric patients:
The recommended dose for a single image sequence is 1.25 mg to 5 mg SPY AGENT GREEN of a 2.5 mg/mL solution. Lower doses may be administered in younger patients and in those with lower body weight. Adjust the amount and type of flush to avoid volume and/or sodium overload.Additional doses may be administered to obtain imaging sequences during the procedure. Do not exceed the maximum total dose of 2 mg/kg.
Administration
Prior to the imaging procedure, draw up the desired dosage of SPY AGENT GREEN solution into appropriate syringes and prepare a 10 mL syringe of 0.9% Sodium Chloride, USP.Administer via a central or peripheral venous line using a three-way stopcock attached to an injection port on the infusion line.
Inject the prepared SPY AGENT GREEN into the line as a tight bolus. Immediately switch the access on the stopcock and inject the prepared flush.
Imaging Instructions for Visualization of Vessels, Blood Flow and Tissue Perfusion
SPY AGENT GREEN may be used with either the SPY ® Elite or PINPOINT ® Fluorescence Imaging Systems or with a FDA cleared or approved imaging device that is specifically intended for fluorescence imaging with indocyanine green.A fluorescence response should be visible in blood vessels within 5 to 15 seconds after injection.
2.2 Recommended Dose and Administration for Visualization of Extrahepatic Biliary Ducts
Dosing and Administration
The recommended single dose of SPY AGENT GREEN for adults and pediatric patients aged 12 to 17 years is 2.5 mg of a 2.5 mg/mL solution administered intravenously as a single dose at least 45 minutes prior to surgery. Additional doses may be administered to obtain imaging sequences during the procedure.
Do not exceed a total dose of 2 mg/kg.Imaging Instructions for Visualization of Extrahepatic Biliary Ducts
SPY AGENT GREEN may be used with the PINPOINT ® Fluorescence Imaging System or with a FDA cleared or approved imaging device that is specifically intended for fluorescence imaging with indocyanine green.Fluorescence is visible in the biliary tree within 45 minutes after injection.
2.3 Recommended Dose and Administration for Visualization of Lymph Nodes and Lymphatic Vessels During Lymphatic Mapping for Cervical and Uterine Tumors
Dosing and Administration
The recommended single dose of SPY AGENT GREEN is 5 mg of a 1.25 mg/mL solution (four 1 mL injections administered interstitially into the cervix, at the three o’ clock and the nine o’clock positions with a superficial (1 mm to 3 mm) and a deep (1 cm to 3 cm) injection at each position).Imaging Instructions for Visualization of Lymph Nodes and Lymphatic Vessels During Lymphatic Mapping
SPY AGENT GREEN may be used with the PINPOINT ® Fluorescence Imaging System or with a FDA cleared or approved imaging device that is specifically intended for fluorescence imaging with indocyanine green.Fluorescent lymphatic vessels and lymph nodes should begin to be visible within 1 minute after injection.
2.4 Reconstitution Instructions
General
- Prepare SPY AGENT GREEN under sterile conditions prior to surgery.
- Inspect the reconstituted solution for particulate matter, the reconstituted solution should be a clear, green solution.
- Use the prepared solution within 6 hours.
- Discard any unused product.
Visualization of Vessels, Blood Flow, and Tissue Perfusion and Visualization of Extrahepatic Biliary Ducts
- Dissolve SPY AGENT GREEN with 10 mL Sterile Water for Injection, USP to form a concentration of 2.5 mg indocyanine green/mL.
Visualization of Lymph Nodes and Lymphatic Vessels During Lymphatic Mapping
- Dissolve SPY AGENT GREEN with 20 mL Sterile Water for Injection, USP to form a concentration of 1.25 mg indocyanine green/mL.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
SPY AGENT GREEN is contraindicated in patients with a history of hypersensitivity to indocyanine green. Reactions have included anaphylaxis [see Warnings and Precautions ( 5.1)] .
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions including anaphylaxis, urticaria, and deaths due to anaphylaxis have been reported following intravenous administration of indocyanine green. [see Adverse Reactions ( 6)]. Always have cardiopulmonary resuscitation personnel and equipment readily available and monitor all patients for hypersensitivity reactions.
5.2 Interference with Radioactive Iodine Uptake Studies
Because SPY AGENT GREEN contains iodine, the iodine-binding capacity of thyroid tissue may be reduced for at least one week following administration. Do not perform radioactive iodine uptake studies for at least a week following administration of SPY AGENT GREEN [ see Drug Interactions ( 7) ].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- Hypersensitivity Reactions [see Warnings and Precautions ( 5.1)] .
The following adverse reactions have been identified during post-approval use of indocyanine green. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Anaphylaxis, urticaria.
-
7 DRUG INTERACTIONS
Effect on Radioactive Iodine Uptake
Because SPY AGENT GREEN contains iodine, the iodine-binding capacity of thyroid tissue may be reduced for at least one week following administration. Do not perform radioactive iodine uptake studies for at least one week following administration of SPY AGENT GREEN [see Warnings and Precautions ( 5.2)]. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of SPY AGENT GREEN in pregnant women. Available data from a very small number of scientific literature studies with indocyanine green use in pregnant women over several decades have not reported any drug associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes. Data from one small study in which indocyanine green was administered intravenously to pregnant women during labor suggest there is no placental transfer of the drug. Animal reproduction studies have not been conducted with indocyanine green.All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
Seventeen cases of indocyanine green use in lactating women have been reported in the scientific literature with no adverse events observed in the breastfed infant. However, there are no data on the presence of indocyanine green in human milk or the effects on milk production. Therefore, the developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for SPY AGENT GREEN and any potential adverse effects on the breastfed infant from SPY AGENT GREEN or from the underlying maternal condition.8.4 Pediatric Use
Use of indocyanine green for visualization of vessels, blood flow and tissue perfusion has been established in pediatric patients one month and older. Pediatric use is supported by published data in 49 pediatric patients who received indocyanine green for assessment of blood flow and tissue perfusion in cardiovascular, vascular and plastic, micro and reconstructive procedures, and by clinical trials in adults. No overall differences in safety or effectiveness have been observed between pediatric patients and adults. The dose range was similar to the effective dose range in adults [See Dosage and Administration ( 2.1)]. The use of indocyanine green for visualization of vessels, blood flow and tissue perfusion has not been established in pediatric patients less than one month of age.
Use of indocyanine green for visualization of extrahepatic biliary ducts has been established in pediatric patients aged 12 to 17 years. Pediatric use is supported by clinical trials in adults in addition to clinical use in pediatric patients. No overall differences in safety or effectiveness have been observed between pediatric patients and adults. The dose range was similar to the effective dose range in adults [ See Dosage and Administration ( 2.2) ]. The use of indocyanine green for visualization of extrahepatic biliary ducts has not been established in pediatric patients less 12 years of age.
The safety and efficacy of indocyanine green for visualization of lymph nodes and lymphatic vessels during lymphatic mapping for cervical and uterine tumors has not been established in pediatric patients.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of SPY AGENT GREEN in visualization of vessels, blood flow, and tissue perfusion, 7 percent were 65 and over, while 1 percent were 75 and over and of the total number of subjects in clinical studies of SPY AGENT GREEN in visualization of lymph nodes and lymphatic vessels during lymphatic mapping, 9 percent were 65 and over, while 2 percent were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Clinical studies of SPY AGENT GREEN in visualization of extrahepatic biliary ducts did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
11 DESCRIPTION
SPY AGENT GREEN (indocyanine green for injection, USP) is a water soluble, optical imaging agent which is reconstituted with Sterile Water for Injection, USP, for intravenous or interstitial use. Each vial contains a sterile, lyophilized green powder containing 25 mg of indocyanine green with not more than 5% sodium iodide.
The chemical name for Indocyanine Green is 1 HBenz[e]indolium, 2-[7-[1,3-dihydro-1,1-dimethyl-3-(4-sulfobutyl)-2H-benz[e] indol-2-ylidene]-1,3,5-heptatrienyl]-1,1-dimethyl-3-(4-sulfobutyl)-, hydroxide, inner salt, sodium salt.
Molecular Formula: C 43H 47N 2NaO 6S 2; Molecular Mass: 774.96 g/mol, with the following structural formula:
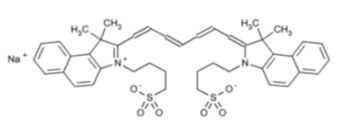
Indocyanine green has a peak spectral absorption at 800 nm. SPY AGENT GREEN has a pH of 5.5 to 7.5 when reconstituted with Sterile Water for Injection, USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
When bound to proteins in plasma or in lymph fluid, indocyanine green absorbs light in the near-infrared region at 805 nm, and emits fluorescence (light) at a slightly longer wavelength, with peak emission at 830 nm. Fluorescence imaging devices provide external energy as near infrared light for ICG to absorb, resulting in excitation of the ICG and the emitted light (fluorescence) is transferred from the field of view to an image on a monitor. These optical properties of indocyanine green are utilized in fluorescence imaging of the micro and macro vasculature, blood flow and tissue perfusion, the extrahepatic biliary ducts, and for lymphatic mapping of lymph nodes and lymphatic vessels.
12.3 Pharmacokinetics
Distribution
Following intravenous injection, indocyanine green binds to plasma proteins (98%) and is largely confined to the intravascular compartment. Indocyanine green undergoes no significant extrahepatic or enterohepatic circulation; simultaneous arterial and venous blood estimations have shown negligible renal, peripheral, lung or cerebro-spinal uptake of the dye. After biliary obstruction, the dye appears in the hepatic lymph, independently of the bile, suggesting that the biliary mucosa is sufficiently intact to prevent diffusion of the dye, though allowing diffusion of bilirubin.Following interstitial injection, indocyanine green is taken up by the lymphatic vessels and lymph nodes.
Elimination
Indocyanine green is taken up from the plasma almost exclusively by the hepatic parenchymal cells and is secreted entirely into the bile.The plasma fractional disappearance rate at a 0.5 mg/kg dose has been reported to be significantly greater in women than in men, although there was no significant difference in the calculated value for clearance.
-
14 CLINICAL STUDIES
Visualization of Lymph Nodes and Lymphatic Vessels During Lymphatic Mapping of Cervical and Uterine Tumors
FILM Study (NCT 02209532)
FILM was a randomized, prospective, multi-center, open-label study in patients with early stage uterine or cervical cancers and no known regional nodal or metastatic disease by standard clinical evaluation. SPY AGENT GREEN and a blue dye comparator were injected into the cervix of patients at the beginning of the operative procedure. A total of 176 patients were randomized to receive either SPY AGENT GREEN followed by blue dye or blue dye followed by SPY AGENT GREEN. A total of four 1 ml injections of a 1.25 mg/ml solution of SPY AGENT GREEN were administered into the cervix at the 3 and 9 o’clock positions with a superficial (1 to 3 mm) and a deep (1 to 3 cm) injection at each position for a total dose of 5 mg per patient. Lymphatic mapping was performed intraoperatively using the PINPOINT ® Fluorescence Imaging System and standard light, followed by excision of tissues identified by SPY AGENT GREEN, blue dye, or the surgeon’s visual and palpation examination. The resected tissues were evaluated by histopathology to confirm presence of lymph nodes. The efficacy of SPY AGENT GREEN and the PINPOINT ® Fluorescence Imaging System in the detection of lymphatic vessels and lymph nodes during lymphatic mapping procedures was determined by the number of histology-confirmed lymph nodes detected by SPY AGENT GREEN and/or the blue dye comparator.Table 1 shows the distribution of resected, confirmed lymph nodes by the presence or absence of SPY AGENT GREEN or blue dye in the modified intent-to-treat population (mITT). Among the confirmed lymph nodes identified, 93% were identified using SPY AGENT GREEN, and 43% were identified using blue dye, a difference of 50% [95% confidence interval 39% to 60%].
Table 1: Distribution of Resected Confirmed Lymph Nodes Detected by SPY AGENT GREEN or Blue Dye (BD) Analysis
populationNodes (n) All Lymph
Nodes
Detected with
SPY AGENT
GREENAll Lymph
Nodes
Detected with
BDLymph
Nodes
Detected
with SPY
AGENT
GREEN OnlyLymph
Nodes
Detected
with BD OnlyLymph
Nodes
Detected
with NeithermITT 513 (476/513)
93%(220/513)
43%(262/513)
51%(6/513)
1%(31/513)
6%Table 2 shows the number of patients with at least one resected, confirmed lymph node and the number of patients with at least one bilateral lymph node pair detected by SPY AGENT GREEN or blue dye. With SPY AGENT GREEN, approximately 97% of patients had at least one resected, confirmed lymph node detected and 73% had at least one bilateral lymph node pair detected, compared with 68% and 28%, respectively, with blue dye [p-values for each analysis <0.0001].
Table 2: Distribution of Patients with at Least One Confirmed Unilateral Lymph Node / Bilateral Pair Detected by SPY AGENT GREEN or Blue Dye (BD) *: patients with at least one resected confirmed lymph node detected unilaterally
**: patients with at least one resected confirmed lymph node detected bilaterallyAnalysis
populationPatients
(n)Patients with
All Lymph
Nodes
Detected with
SPY AGENT
GREENPatients with
All Lymph
Nodes
Detected with
BDPatients with
Lymph
Nodes
Detected
with SPY
AGENT
GREEN OnlyPatients with
Lymph
Nodes
Detected
with BD OnlyPatients with
Lymph
Nodes
Detected
with NeithermITT
Unilateral *172 (167/172)
97%(118/172)
68%(51/172)
30%(2/172)
1%(3/172)
3%mITT Bilateral ** (126/172)
73%(49/172)
28%(79/172)
46%(2/172)
1%(44/172)
26% -
16 HOW SUPPLIED/STORAGE AND HANDLING
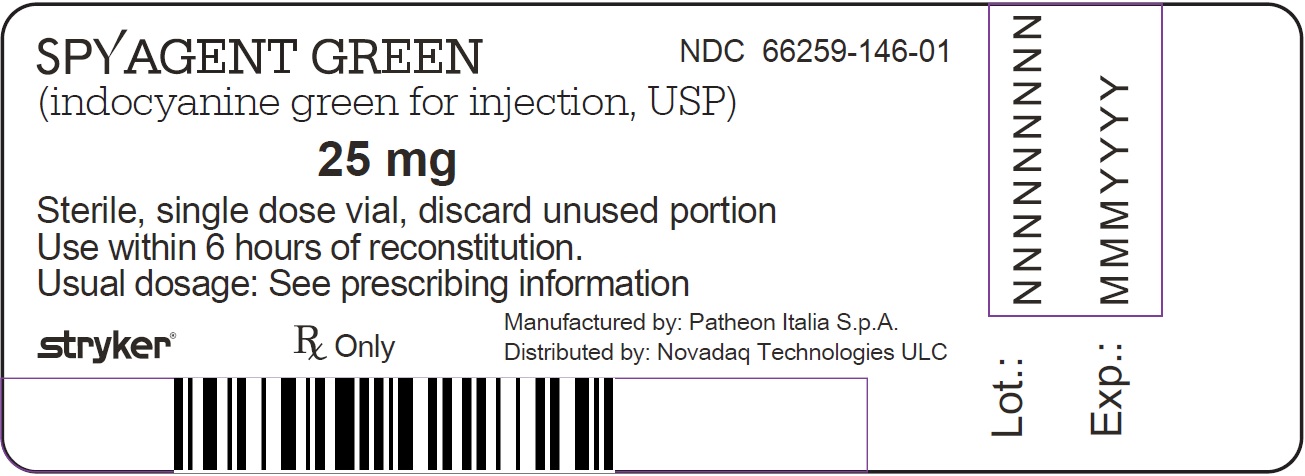
NDC: 66259-146-01
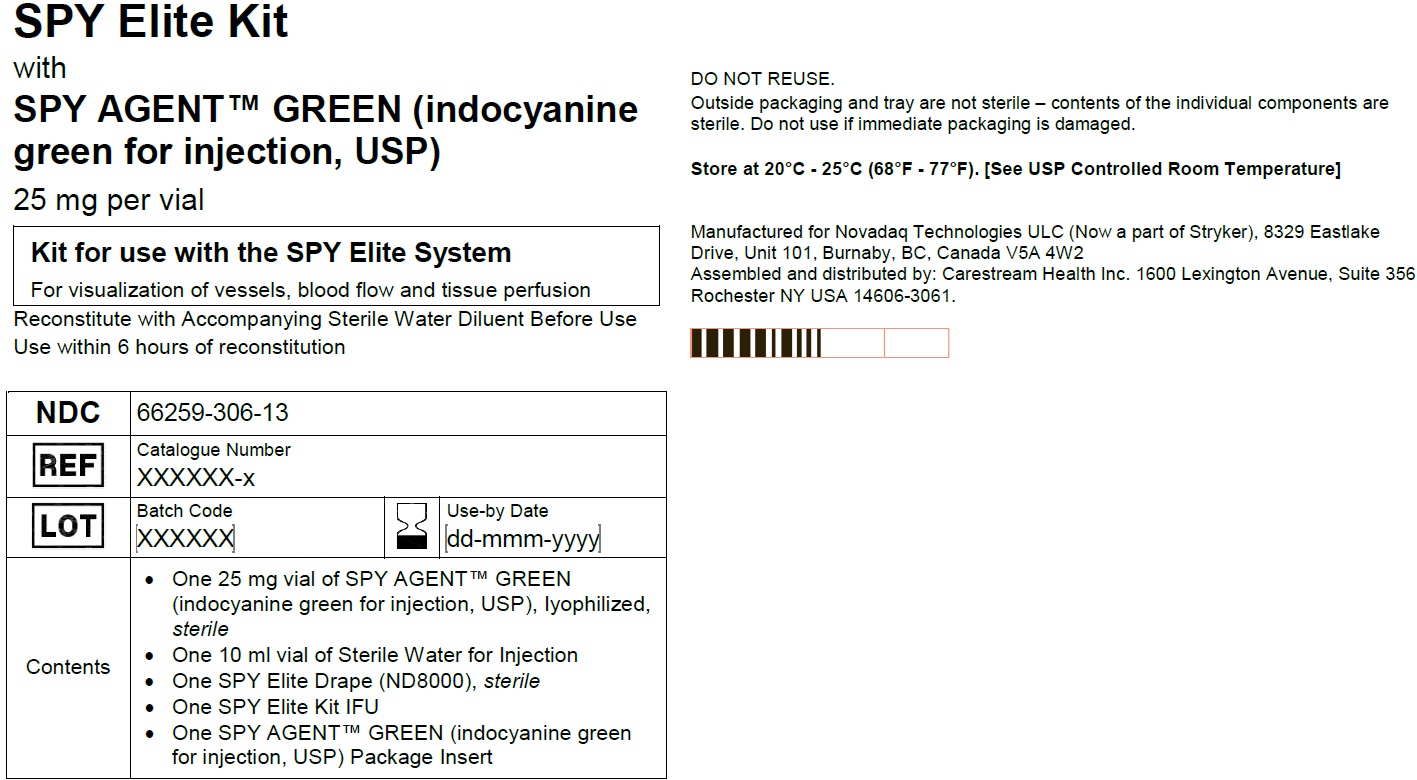
SPY AGENT GREEN (indocyanine green for injection, USP) is supplied as 25 mg of lyophilized powder in a 20 mL single patient use vial in the following configurations:Spy Elite Kit
NDC: 66259-306-13
Kit for use with SPY Elite System
For visualization of vessels, blood flow, and tissue perfusion
One 25 mg SPY AGENT GREEN (Indocyanine Green for Injection, USP) vial,
One 10 mL Sterile Water for Injection, USP plastic vial,
One ND8000 sterile drape
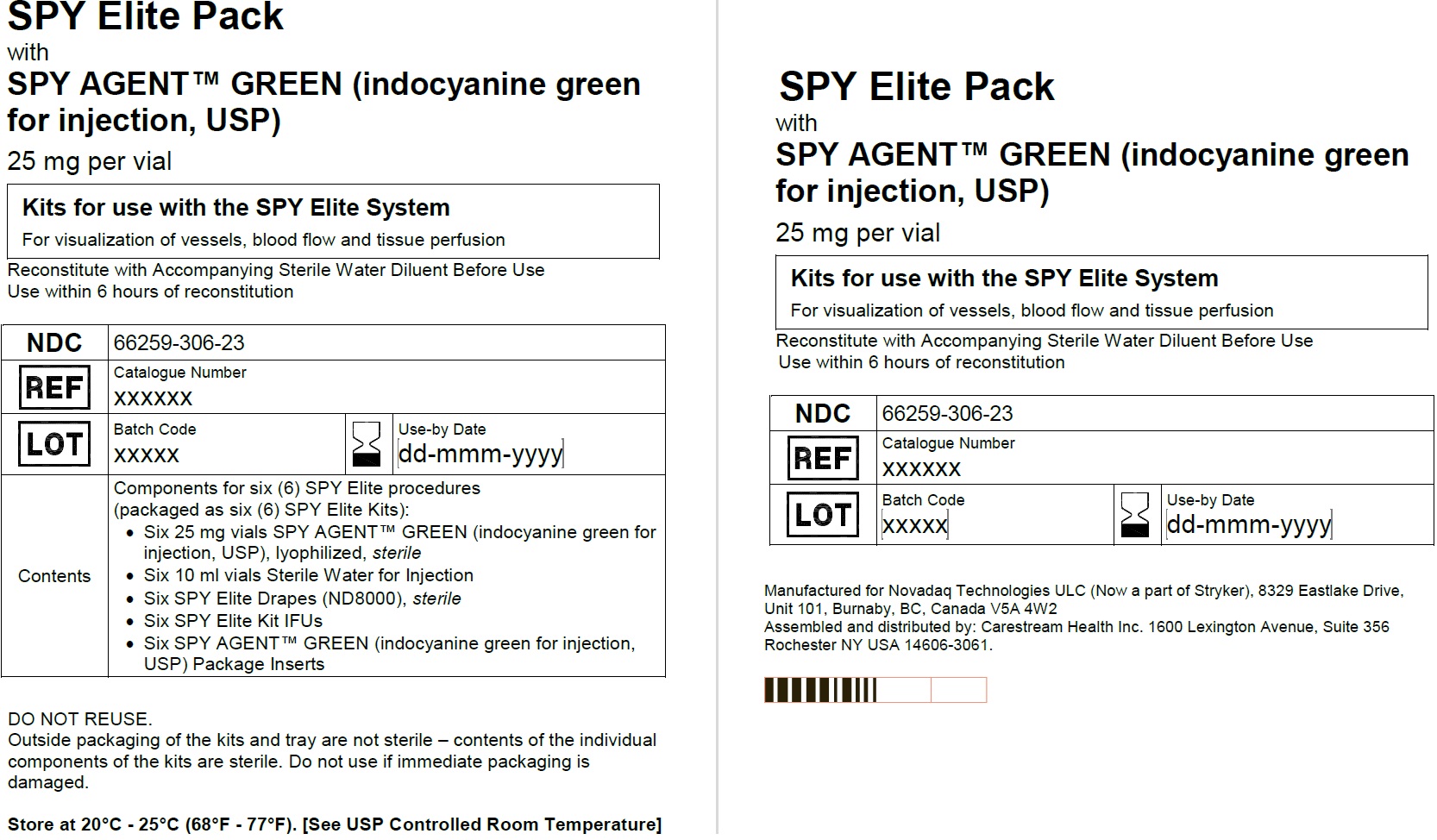
Spy Elite Pack
NDC: 66259-306-23
6 SPY Elite kits:
Six 25 mg SPY AGENT GREEN (Indocyanine Green for Injection, USP) vials,
Six 10 mL Sterile Water for Injection, USP plastic vials,
Six ND8000 sterile drapes
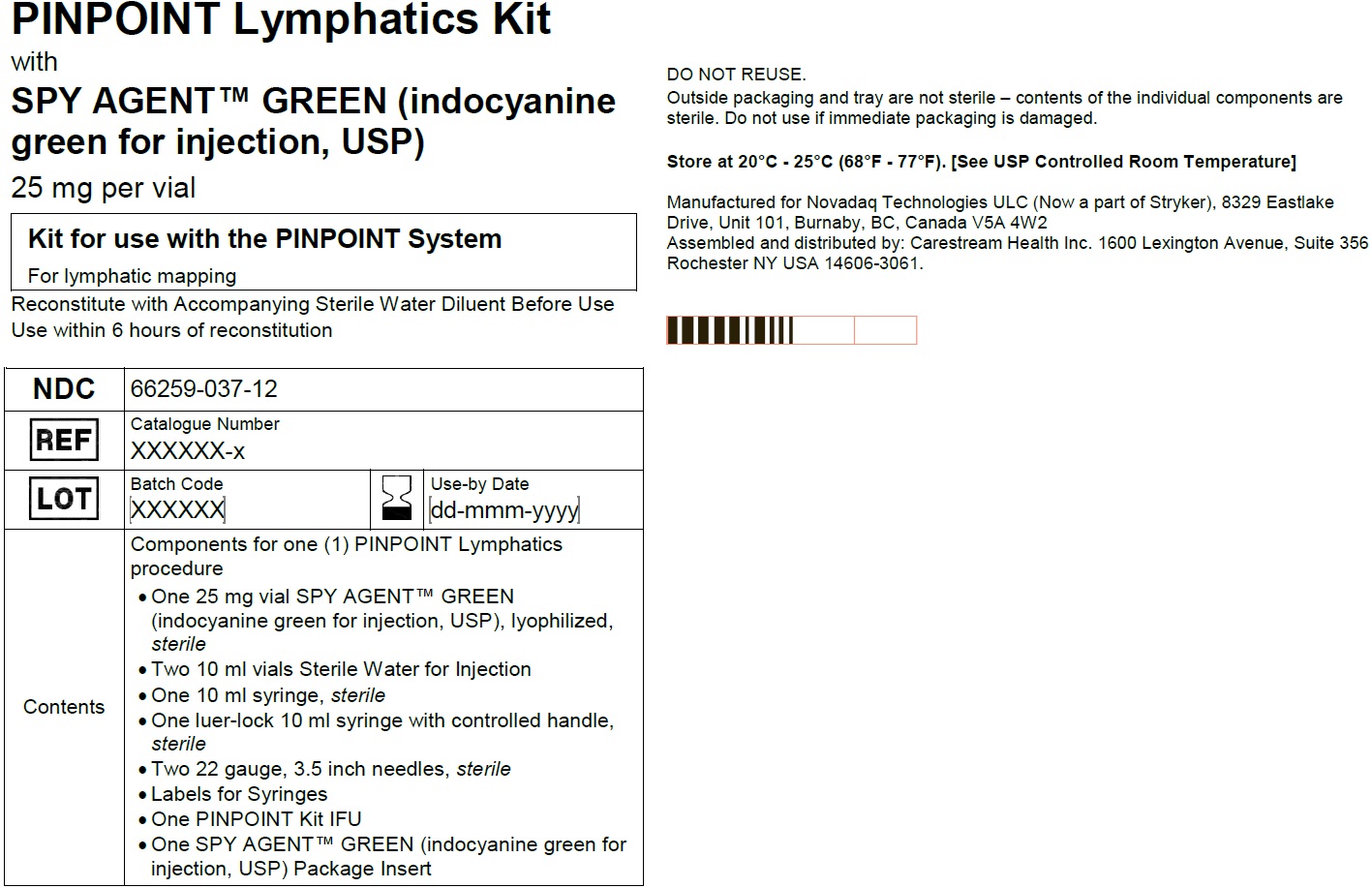
PINPOINT Lymphatics Kit
NDC: 66259-037-12
Kit for use with PINPOINT System
For lymphatic mapping
One 25 mg SPY AGENT GREEN (Indocyanine Green for Injection, USP) vial,
Two 10 mL Sterile Water for Injection, USP plastic vials,
One 10 ml syringe (sterile),
One luer-lock 10 ml syringe with controlled handle (sterile), Two spinal needles 22G, 3.5 inch (sterile),
Labels for syringes
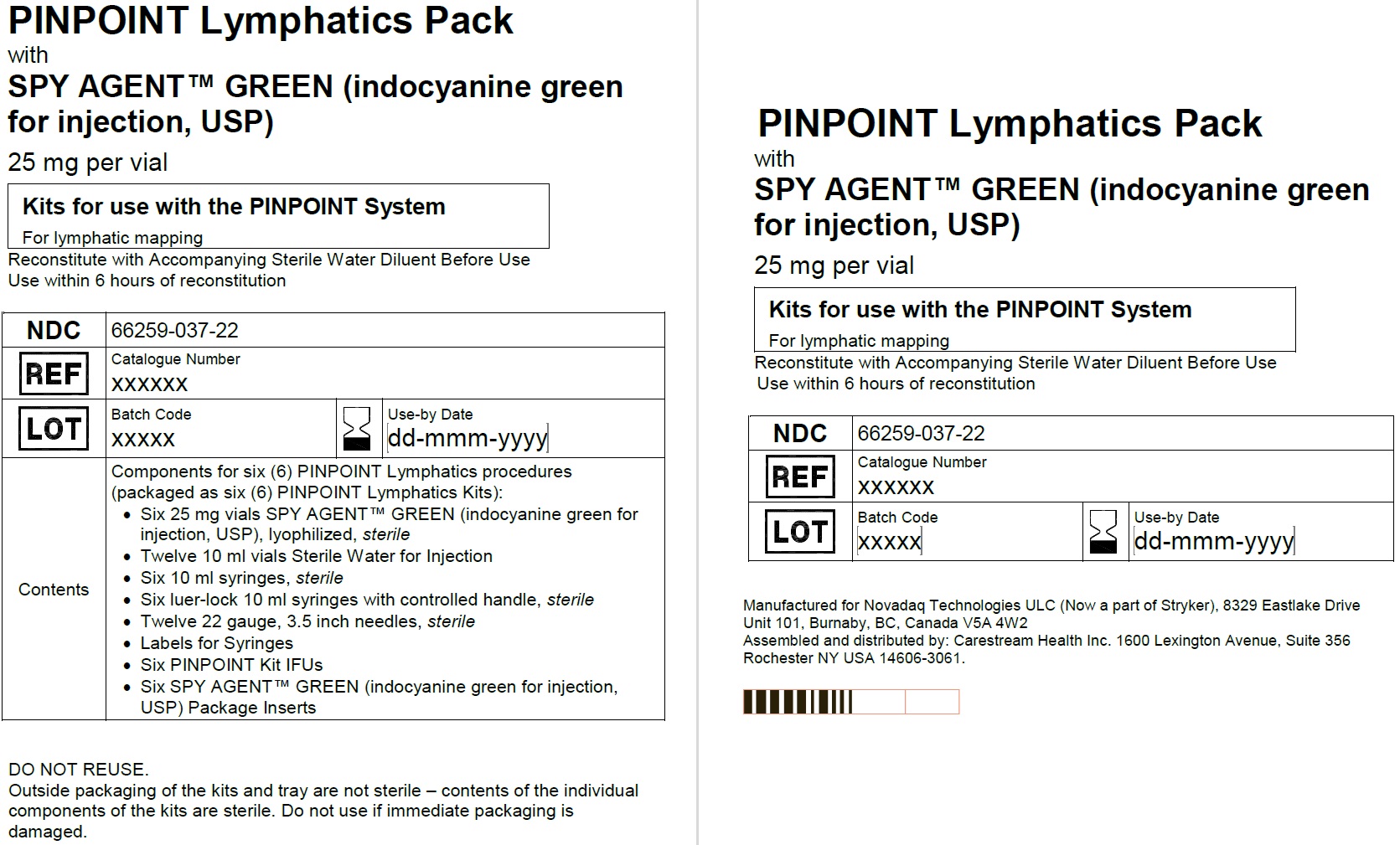
PINPOINT Lymphatics Pack
NDC: 66259-037-22
6 PINPOINT lymphatics kits
Six 25 mg SPY AGENT GREEN (Indocyanine Green for Injection, USP) vials,
Twelve 10 mL Sterile Water for Injection, USP plastic vials,
Six 10 ml syringes (sterile),
Six luer-lock 10 ml syringes with controlled handle (sterile), Twelve spinal needles 22G, 3.5 inch (sterile)
Labels for syringes
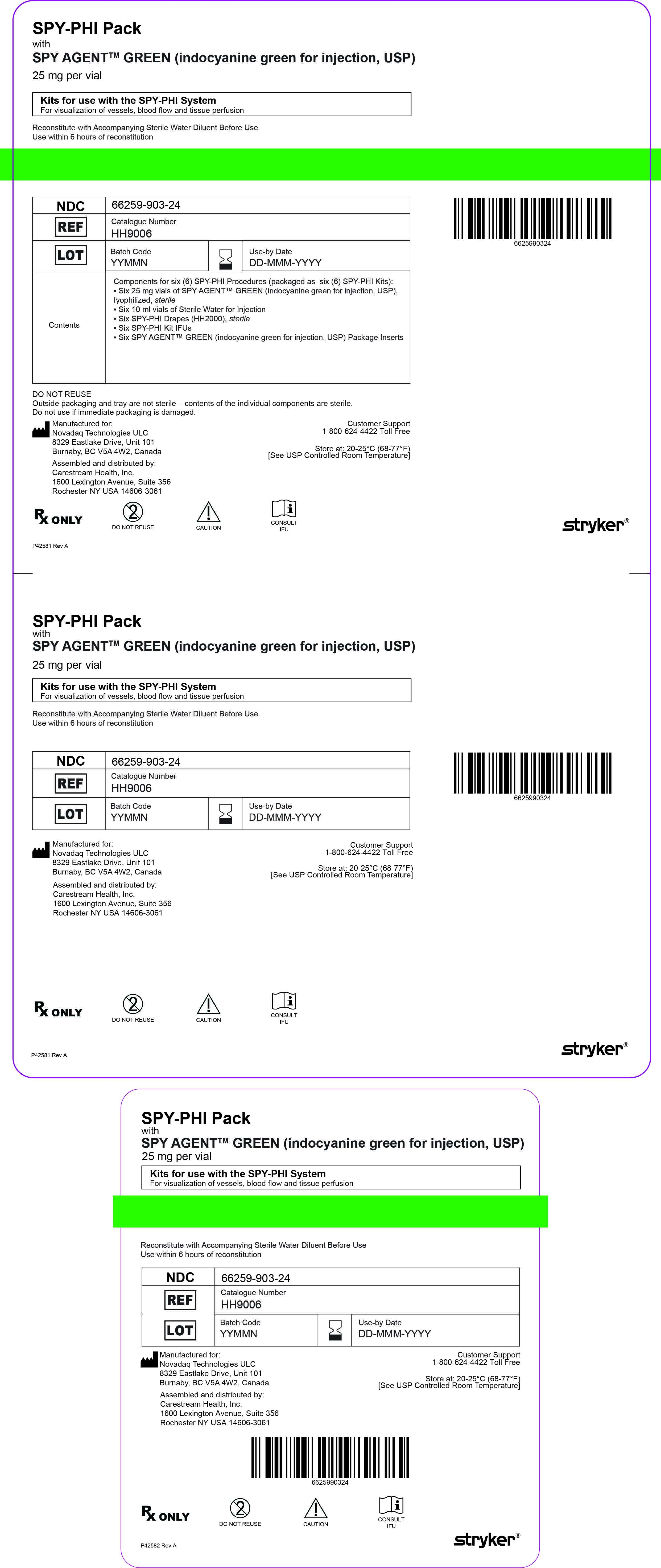
SPY-PHI Kit
NDC: 66259-903-14
Kit for use with SPY-PHI System
For visualization of vessels, blood flow, and tissue perfusion
One 25 mg SPY AGENT GREEN (Indocyanine Green for Injection, USP) vial,
One 10 mL Sterile Water for Injection, USP plastic vial,
One SPY-PHI (HH2000) sterile drape
SPY-PHI Pack
NDC: 66259-903-24
6 SPY-PHI kits:
Six 25 mg SPY AGENT GREEN (Indocyanine Green for Injection, USP) vials,
Six 10 mL Sterile Water for Injection, USP plastic vials,
Six SPY-PHI (HH2000) sterile drapes
SPY Minimally Invasive Surgery (SPY-MIS) Kit
NDC: 66259-936-15
Kit for use with Advanced Imaging Modality (AIM) and PINPOINT Systems
For visualization of vessels, blood flow, and tissue perfusion
For visualization of extrahepatic biliary ducts
One 25 mg SPY AGENT GREEN (Indocyanine Green for Injection, USP) vial,
One 10 mL Sterile Water for Injection, USP plastic vial,
Two 3 ml syringes (sterile),
Two 10 ml syringes (sterile),
One 3-way stopcock (sterile),
Two 18G, 1 inch needles (sterile),
Labels for syringes
SPY Minimally Invasive Surgery (SPY-MIS) Pack
NDC: 66259-936-25
6 SPY-MIS kits:
Six 25 mg SPY AGENT GREEN (Indocyanine Green for Injection, USP) vials,
Six 10 mL Sterile Water for Injection, USP plastic vials,
Twelve 3 ml syringes (sterile),
Twelve 10 ml syringes (sterile),
Six 3-way stopcocks (sterile),
Twelve 18G, 1 inch needles (sterile),
Labels for syringes
SPY Lymphatics Kit
NDC: 66259-937-16
Kit for use with the Advanced Imaging Modality (AIM) L10 and L11 Light Sources and 1688 Camera System, and the PINPOINT System
For lymphatic mapping
One 25 mg SPY AGENT GREEN (Indocyanine Green for Injection, USP) vial,
Two 10 mL Sterile Water for Injection, USP plastic vials,
One 10 ml syringe (sterile),
One luer-lock 10 ml syringe with controlled handle (sterile), Two spinal needles 22G, 3.5 inch (sterile),
Labels for syringes
SPY Lymphatics Pack
NDC: 66259-937-26
6 SPY Lymphatics kits:
Six 25 mg SPY AGENT GREEN (Indocyanine Green for Injection, USP) vials,
Twelve 10 mL Sterile Water for Injection, USP plastic vials,
Six 10 ml syringes (sterile),
Six luer-lock 10 ml syringes with controlled handle (sterile), Twelve spinal needles 22G, 3.5 inch (sterile)
Labels for syringes
NDC: 63323-185-10 (or NDC: 0409-4887-10) Sterile Water for Injection, USP, 10 mL fill in 10 mL plastic vials.
STORAGE: Store at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature].
PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise patients to seek medical attention for reactions following injection of SPY AGENT GREEN such as difficulty breathing, swollen tongue or throat, skin reactions including hives, itching and flushed or pale skin, low blood pressure, a weak and rapid pulse and other symptoms or signs of an anaphylactic reaction [see Warnings and Precautions ( 5.1)] .
Manufactured by:
Patheon Italia S.p.A.
Ferentino (FR), ItalyDistributed by:
Novadaq Technologies ULC
8329 Eastlake Drive, Unit 101,
Burnaby, BC, Canada V5A 4W2Sterile Water for Injection, USP is manufactured by:
Fresenius Kabi USA, LLC
Grand Island, NY 14072 U.S.A.
or
Hospira, Inc.
Rocky Mount, NC 27804 U.S.A. - PRINCIPAL DISPLAY PANEL - NDC: 66259-306-13 - SPY Elite Kit Label
- PRINCIPAL DISPLAY PANEL - NDC: 66259-306-23 - SPY Elite Pack Label
- PRINCIPAL DISPLAY PANEL - NDC: 66259-037-12 - PINPOINT Lymphatics Kit Label
- PRINCIPAL DISPLAY PANEL - NDC: 66259-037-22 - PINPOINT Lymphatics Pack Label
- PRINCIPAL DISPLAY PANEL - NDC: 66259-146-01 - SPY Agent Green 25 mg Vial Label
- PRINCIPAL DISPLAY PANEL - NDC: 66259-903-14 - SPY-PHI Kit Label
- PRINCIPAL DISPLAY PANEL - NDC: 66259-903-24 - SPY-PHI Pack Label
- PRINCIPAL DISPLAY PANEL - NDC: 66259-936-15 - SPY-MIS Kit Label
- PRINCIPAL DISPLAY PANEL - NDC: 66259-936-25 - SPY-MIS Pack Label
- PRINCIPAL DISPLAY PANEL - NDC: 66259-937-16 - SPY Lymphatics Kit Label
- PRINCIPAL DISPLAY PANEL - NDC: 66259-937-26 - SPY Lymphatics Pack Label
-
INGREDIENTS AND APPEARANCE
SPY-PHI KIT
indocyanine green kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66259-903 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66259-903-14 1 in 1 KIT 09/10/2019 2 NDC: 66259-903-24 6 in 1 CASE 09/10/2019 2 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1 Part 2 1 VIAL, PLASTIC 10 mL Part 1 of 2 SPY AGENT GREEN
indocyanine green injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 66259-146 Route of Administration INTERSTITIAL, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INDOCYANINE GREEN (UNII: IX6J1063HV) (INDOCYANINE GREEN ACID FORM - UNII:C4V974V932) INDOCYANINE GREEN 25 mg Inactive Ingredients Ingredient Name Strength SODIUM IODIDE (UNII: F5WR8N145C) Product Characteristics Color green (OLIVE BROWN, DARK GREEN, BLUE GREEN, DARK BLUE OR BLACK LYOPHILIZED POWDER FREE FROM VISIBLE CONTAMINATION) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66259-146-01 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211580 Part 2 of 2 STERILE WATER
water injectionProduct Information Item Code (Source) NDC: 0409-4887 Route of Administration INTRAVENOUS, INTRAVASCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018801 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211580 09/10/2019 SPY ELITE KIT
indocyanine green kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66259-306 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66259-306-13 1 in 1 KIT 11/21/2018 2 NDC: 66259-306-23 6 in 1 CASE 11/21/2018 2 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, PLASTIC 10 mL Part 2 1 VIAL 1 Part 1 of 2 STERILE WATER
water injectionProduct Information Item Code (Source) NDC: 0409-4887 Route of Administration INTRAVENOUS, INTRAVASCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018801 Part 2 of 2 SPY AGENT GREEN
indocyanine green injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 66259-146 Route of Administration INTRAVENOUS, INTERSTITIAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INDOCYANINE GREEN (UNII: IX6J1063HV) (INDOCYANINE GREEN ACID FORM - UNII:C4V974V932) INDOCYANINE GREEN 25 mg Product Characteristics Color green (Olive brown, dark green, blue green, dark blue or black lyophilized powder free from visible contamination) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66259-146-01 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211580 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211580 11/21/2018 SPY LYMPHATICS KIT
indocyanine green kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66259-937 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66259-937-16 1 in 1 KIT; Type 0: Not a Combination Product 04/13/2020 2 NDC: 66259-937-26 6 in 1 CASE 04/13/2020 2 1 in 1 KIT; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 VIAL, PLASTIC 20 mL Part 2 1 VIAL 1 Part 1 of 2 STERILE WATER
water injectionProduct Information Item Code (Source) NDC: 0409-4887 Route of Administration INTRAVENOUS, INTERSTITIAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018801 Part 2 of 2 SPY AGENT GREEN
indocyanine green injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 66259-146 Route of Administration INTRAVENOUS, INTERSTITIAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INDOCYANINE GREEN (UNII: IX6J1063HV) (INDOCYANINE GREEN ACID FORM - UNII:C4V974V932) INDOCYANINE GREEN 25 mg Inactive Ingredients Ingredient Name Strength SODIUM IODIDE (UNII: F5WR8N145C) Product Characteristics Color green (OLIVE BROWN, DARK GREEN, DARK BLUE OR BLACK LYOPHILIZED POWDER FREE FROM VISIBLE CONTAMINATION) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66259-146-01 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211580 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211580 04/13/2020 PINPOINT LYMPHATICS KIT
indocyanine green kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66259-037 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66259-037-12 1 in 1 KIT 11/21/2018 2 NDC: 66259-037-22 6 in 1 CASE 11/21/2018 2 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 VIAL, PLASTIC 20 mL Part 2 1 VIAL 1 Part 1 of 2 STERILE WATER
water injectionProduct Information Item Code (Source) NDC: 0409-4887 Route of Administration INTRAVENOUS, INTERSTITIAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018801 Part 2 of 2 SPY AGENT GREEN
indocyanine green injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 66259-146 Route of Administration INTRAVENOUS, INTERSTITIAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INDOCYANINE GREEN (UNII: IX6J1063HV) (INDOCYANINE GREEN ACID FORM - UNII:C4V974V932) INDOCYANINE GREEN 25 mg Product Characteristics Color green (Olive brown, dark green, blue green, dark blue or black lyophilized powder free from visible contamination) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66259-146-01 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211580 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211580 11/21/2018 SPY-MIS KIT
indocyanine green kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66259-936 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66259-936-15 1 in 1 KIT 09/10/2019 2 NDC: 66259-936-25 6 in 1 CASE 09/10/2019 2 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1 Part 2 1 VIAL, PLASTIC 10 mL Part 1 of 2 SPY AGENT GREEN
indocyanine green injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 66259-146 Route of Administration INTRAVENOUS, INTERSTITIAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INDOCYANINE GREEN (UNII: IX6J1063HV) (INDOCYANINE GREEN ACID FORM - UNII:C4V974V932) INDOCYANINE GREEN 25 mg Inactive Ingredients Ingredient Name Strength SODIUM IODIDE (UNII: F5WR8N145C) Product Characteristics Color green (OLIVE BROWN, DARK GREEN, BLUE GREEN, DARK BLUE OR BLACK LYOPHLIZED POWDER FREE FROM VISIBLE CONTAMINATION) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66259-146-01 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211580 Part 2 of 2 STERILE WATER
water injectionProduct Information Item Code (Source) NDC: 0409-4887 Route of Administration INTRAVENOUS, INTERSTITIAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018801 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211580 09/10/2019 Labeler - Novadaq Technologies Inc (200571698)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.