IBUPROFEN by Pharmaceutical Associates, Inc. / L. Perrigo Company IBUPROFEN suspension
IBUPROFEN by
Drug Labeling and Warnings
IBUPROFEN by is a Prescription medication manufactured, distributed, or labeled by Pharmaceutical Associates, Inc., L. Perrigo Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
Cardiovascular Thrombotic Events
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use (see WARNINGS and PRECAUTIONS).
- Ibuprofen Oral Suspension is contraindicated in the setting of coronary artery bypass graft (CABG) surgery (see CONTRAINDICATIONS and WARNINGS).
Gastrointestinal Risk
- NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events (see WARNINGS).
-
DESCRIPTION
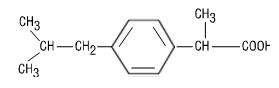
The active ingredient in Ibuprofen Oral Suspension USP, 100 mg/5 mL is ibuprofen, which is a member of the propionic acid group of nonsteroidal anti-inflammatory drugs (NSAIDs). Ibuprofen is a racemic mixture of [+]S- and [-]R-enantiomers. It is a white to off-white crystalline powder, with a melting point of 74° to 77°C. It is practically insoluble in water (<0.1 mg/mL), but readily soluble in organic solvents such as ethanol and acetone. Ibuprofen has a pKa of 4.43±0.03 and an n-octanol/water partition coefficient of 11.7 at pH 7.4. The chemical name for ibuprofen is (±)-2-(p-isobutylphenyl) propionic acid. The molecular weight of ibuprofen is 206.28. Its molecular formula is C 13H 18O 2 and it has the following structural formula:
Ibuprofen Oral Suspension is a sweetened, orange colored, berry flavored suspension containing 100 mg of ibuprofen in 5 mL (20 mg/mL). Inactive ingredients include: anhydrous citric acid, artificial berry flavor, butylparaben, D&C red #33, FD&C yellow #6, glycerin, high fructose corn syrup, hypromellose, polysorbate 80, propylene glycol, purified water, sodium benzoate, sorbitol solution, xanthan gum.
-
CLINICAL PHARMACOLOGY
Pharmacodynamics
Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that possesses anti-inflammatory, analgesic and antipyretic activity. Its mode of action, like that of other NSAIDs, is not completely understood, but may be related to prostaglandin synthetase inhibition. After absorption of the racemic ibuprofen, the [-]R-enantiomer undergoes interconversion to the [+]S-form. The biological activities of ibuprofen are associated with the [+]S-enantiomer.
Pharmacokinetics
Ibuprofen is a racemic mixture of [-]R-and [+]S-isomers. In vivo and in vitro studies indicate that the [+]S-isomer is responsible for clinical activity. The [-]R-form, while thought to be pharmacologically inactive, is slowly and incompletely (~60%) interconverted into the active [+]S species in adults. The degree of interconversion in children is unknown, but is thought to be similar. The [-]R-isomer serves as a circulating reservoir to maintain levels of active drug. Ibuprofen is well absorbed orally, with less than 1% being excreted in the urine unchanged. It has a biphasic elimination time curve with a plasma half-life of approximately 2 hours. Studies in febrile children have established the dose-proportionality of 5 and 10 mg/kg doses of ibuprofen. Studies in adults have established the dose-proportionality of ibuprofen as a single oral dose from 50 to 600 mg for total drug and up to 1200 mg for free drug.
Absorption
In vivo studies indicate that ibuprofen is well absorbed orally from the suspension formulation, with peak plasma levels usually occurring within 1 to 2 hours (see Table 1).
Table 1 Pharmacokinetic Parameters of Ibuprofen Oral Suspension [Mean values (% coefficient of variation)] Dose 200 mg (2.8 mg/kg) in Adults 10 mg/kg in Febrile Children Formulation Suspension Suspension Legend: AUCinf = Area-under-the-curve to infinity
Tmax = Time-to-peak plasma concentration
Cmax = Peak plasma concentration
Cl/F = Clearance divided by fraction of drug absorbedNumber of Patients 24 18 AUCinf (µg∙h/mL) 64
(27%)155
(24%)Cmax (µg/mL) 19
(22%)55
(23%)Tmax (h) 0.79
(69%)0.97
(57%)Cl/F (mL/h/kg) 45.6
(22%)68.6
(22%)Antacids
A bioavailability study in adults has shown that there was no interference with the absorption of ibuprofen when given in conjunction with an antacid containing both aluminum hydroxide and magnesium hydroxide.
H-2 Antagonists
In studies with human volunteers, coadministration of cimetidine or ranitidine with ibuprofen had no substantive effect on ibuprofen serum concentrations.
Food Effects
Absorption is most rapid when ibuprofen is given under fasting conditions. Administration of ibuprofen oral suspension with food affects the rate but not the extent of absorption. When taken with food, T max is delayed by approximately 30 to 60 minutes, and peak levels are reduced by approximately 30 to 50%.
Distribution
Ibuprofen, like most drugs of its class, is highly protein bound (>99% bound at 20 µg/mL). Protein binding is saturable and at concentrations >20 µg/mL binding is non-linear. Based on oral dosing data there is an age- or fever-related change in volume of distribution for ibuprofen. Febrile children <11 years old have a volume of approximately 0.2 L/kg while adults have a volume of approximately 0.12 L/kg. The clinical significance of these findings is unknown.
Metabolism
Following oral administration, the majority of the dose was recovered in the urine within 24 hours as the hydroxy-(25%) and carboxypropyl-(37%) phenylpropionic acid metabolites. The percentages of free and conjugated ibuprofen found in the urine were approximately 1% and 14%, respectively. The remainder of the drug was found in the stool as both metabolites and unabsorbed drug.
Elimination
Ibuprofen is rapidly metabolized and eliminated in the urine. The excretion of ibuprofen is virtually complete 24 hours after the last dose. It has a biphasic plasma elimination time curve with a half-life of approximately 2.0 hours. There is no difference in the observed terminal elimination rate or half-life between children and adults, however, there is an age- or fever-related change in total clearance. This suggests that the observed change in clearance is due to changes in the volume of distribution of ibuprofen (see Table 1 for Cl/F values).
Clinical Studies
Controlled clinical trials comparing doses of 5 and 10 mg/kg ibuprofen oral suspension and 10-15 mg/kg of acetaminophen elixir have been conducted in children 6 months to 12 years of age with fever primarily due to viral illnesses. In these studies there were no differences between treatments in fever reduction for the first hour and maximum fever reduction occurred between 2 and 4 hours. Response after 1 hour was dependent on both the level of temperature elevation as well as the treatment. In children with baseline temperatures at or below 102.5°F both ibuprofen doses and acetaminophen were equally effective in their maximum effect. In children with temperatures above 102.5°F, the ibuprofen 10 mg/kg dose was more effective. By 6 hours, children treated with ibuprofen 5 mg/kg tended to have recurrence of fever, whereas children treated with ibuprofen 10 mg/kg still had significant fever reduction at 8 hours. In control groups treated with 10 mg/kg acetaminophen, fever reduction resembled that seen in children treated with 5 mg/kg of ibuprofen, with the exception that temperature elevation tended to return 1-2 hours earlier.
In patients with primary dysmenorrhea, ibuprofen has been shown to reduce elevated levels of prostaglandin activity in the menstrual fluid and to reduce testing and active intrauterine pressure, as well as the frequency of uterine contractions. The probable mechanism of action is to inhibit prostaglandin synthesis rather than simply to provide analgesia.
-
INDICATIONS AND USAGE
Carefully consider the potential benefits and risks of Ibuprofen Oral Suspension and other treatment options before deciding to use Ibuprofen Oral Suspension. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).
In Pediatric Patients, Ibuprofen Oral Suspension is indicated:
- For reduction of fever in patients aged 6 months up to 2 years of age.
- For relief of mild to moderate pain in patients aged 6 months up to 2 years of age.
- For relief of signs and symptoms of juvenile arthritis.
In Adults, Ibuprofen Oral Suspension is indicated:
- For treatment of primary dysmenorrhea.
- For relief of the signs and symptoms of rheumatoid arthritis and osteoarthritis.
Since there have been no controlled trials to demonstrate whether there is any beneficial effect or harmful interaction with the use of ibuprofen in conjunction with aspirin, the combination cannot be recommended (see PRECAUTIONS – Drug Interactions).
-
CONTRAINDICATIONS
Ibuprofen Oral Suspension is contraindicated in patients with known hypersensitivity to ibuprofen.
Ibuprofen Oral Suspension should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients (see WARNINGS - Anaphylactoid Reactions and PRECAUTIONS - Preexisting Asthma).
Ibuprofen Oral Suspension is contraindicated in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).
-
WARNINGS
CARDIOVASCULAR EFFECTS
Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, including myocardial infarction(MI) and stroke, which can be fatal. Based on available data, it is unclear that the risk for CV thrombotic events is similar for all NSAIDs. The relative increase in serious CV thrombotic events over baseline conferred by NSAID use appears to be similar in those with and without known CV disease or risk factors for CV disease. However, patients with known CV disease or risk factors had a higher absolute incidence of excess serious CV thrombotic events, due to their increased baseline rate. Some observational studies found that this increased risk of serious CV thrombotic events began as early as the first weeks of treatment. The increase in CV thrombotic risk has been observed most consistently at higher doses.
To minimize the potential risk for an adverse CV event in NSAID-treated patients, use the lowest effective dose for the shortest duration possible. Physicians and patients should remain alert for the development of such events, throughout the entire treatment course, even in the absence of previous CV symptoms. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID, such as ibuprofen, increases the risk of serious gastrointestinal (GI) events (see WARNINGS).
Status Post Coronary Artery Bypass Graft (CABG) Surgery
Two large, controlled clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10-14 days following CABG surgery found an increased incidence of myocardial infarction and stroke. NSAIDs are contraindicated in the setting of CABG (see CONTRAINDICATIONS).
Post-MI Patients
Observational studies conducted in the Danish National Registry have demonstrated that patients treated with NSAIDs in the post-MI period were at increased risk of reinfarction, CV-related death, and all-cause mortality beginning in the first week of treatment. In this same cohort, the incidence of death in the first year post MI was 20 per 100 person years in NSAID-treated patients compared to 12 per 100 person years in non-NSAID exposed patients. Although the absolute rate of death declined somewhat after the first year post-MI, the increased relative risk of death in NSAID users persisted over at least the next four years of follow-up.
Avoid the use of Ibuprofen Oral Suspension in patients with a recent MI unless the benefits are expected to outweigh the risks of recurrent CV thrombotic events. If Ibuprofen Oral Suspension is used in patients with a recent MI, monitor patients for signs of cardiac ischemia.
Hypertension
NSAIDs, including Ibuprofen Oral Suspension, can lead to onset of new hypertension or worsening of pre-existing hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including Ibuprofen Oral Suspension, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
Heart Failure and Edema
The Coxib and traditional NSAID Trialists' Collaboration meta-analysis of randomized controlled trials demonstrated an approximately two-fold increase in hospitalizations for heart failure in COX-2 selective-treated patients and nonselective NSAID-treated patients compared to placebo-treated patients. In a Danish National Registry study of patients with heart failure, NSAID use increased the risk of MI, hospitalization for heart failure, and death.
Additionally, fluid retention and edema have been observed in some patients treated with NSAIDs. Use of ibuprofen may blunt the CV effects of several therapeutic agents used to treat these medical conditions [e.g., diuretics, ACE inhibitors, or angiotensin receptor blockers (ARBs)] (see Drug Interactions).
Avoid the use of Ibuprofen Oral Suspension in patients with severe heart failure unless the benefits are expected to outweigh the risk of worsening heart failure. If Ibuprofen Oral Suspension is used in patients with severe heart failure, monitor patients for signs of worsening heart failure.
Gastrointestinal Effects – Risk of Ulceration, Bleeding, and Perforation
NSAIDs, including Ibuprofen Oral Suspension, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3-6 months, and in about 2-4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.
NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population.
To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
Renal Effects
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a nonsteroidal anti-inflammatory drug may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
Advanced Renal Disease
No information is available from controlled clinical studies regarding the use of Ibuprofen Oral Suspension in patients with advanced renal disease. Therefore, treatment with Ibuprofen Oral Suspension is not recommended in these patients with advanced renal disease. If Ibuprofen Oral Suspension therapy must be initiated, close monitoring of the patient's renal function is advisable.
Anaphylactoid Reactions
As with other NSAIDs, anaphylactoid reactions may occur in patients without known prior exposure to Ibuprofen Oral Suspension. Ibuprofen Oral Suspension should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs (see CONTRAINDICATIONS and PRECAUTIONS - Preexisting Asthma). Emergency help should be sought in cases where an anaphylactoid reaction occurs.
Skin Reactions
NSAIDs, including Ibuprofen Oral Suspension, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
-
PRECAUTIONS
General
Ibuprofen Oral Suspension cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.
The pharmacological activity of Ibuprofen Oral Suspension in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.
Hepatic Effects
Borderline evaluations of one or more liver tests may occur in up to 15% of patients taking NSAIDs including Ibuprofen Oral Suspension. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continuing therapy. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes have been reported.
A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with Ibuprofen Oral Suspension. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), Ibuprofen Oral Suspension should be discontinued.
Hematological Effects
Anemia is sometimes seen in patients receiving NSAIDs, including Ibuprofen Oral Suspension. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including Ibuprofen Oral Suspension, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.
In two postmarketing clinical studies the incidence of a decreased hemoglobin level was greater than previously reported. Decrease in hemoglobin of 1 gram or more was observed in 17.1% of 193 patients on 1600 mg ibuprofen daily (osteoarthritis), and in 22.8% of 189 patients taking 2400 mg of ibuprofen daily (rheumatoid arthritis). Positive stool occult blood tests and elevated serum creatinine levels were also observed in these studies.
NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving Ibuprofen Oral Suspension who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.
Preexisting Asthma
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm, which can be fatal. Since cross reactivity, including bronchospasm, between aspirin and other nonsteroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, Ibuprofen Oral Suspension should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma.
Aseptic Meningitis
Aseptic meningitis, with fever and coma, has been observed on rare occasions in patients on ibuprofen therapy. Although it is probably more likely to occur in patients with systemic lupus erythematosus and related connective tissue diseases, it has been reported in patients who do not have an underlying chronic disease.
Diabetics
Ibuprofen Oral Suspension contains 270 mg high fructose corn syrup and 0.83 calories per mL, or 1350 mg high fructose corn syrup and 4.15 calories per teaspoonful, which should be taken into consideration when treating diabetic patients with this product.
Information for Patients
Patients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.
- Advise patients to be alert for the symptoms of cardiovascular thrombotic events, including chest pain, shortness of breath, weakness, or slurring of speech, and to report any of these symptoms to their health care provider immediately (see WARNINGS).
- Ibuprofen Oral Suspension, like other NSAIDs, can cause GI discomfort and, rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization or even death. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing any indicative signs or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up (see WARNINGS - Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation).
- Ibuprofen Oral Suspension, like other NSAIDs, can cause serious skin side effects such as exfoliative dermatitis, SJS, and TEN, which may result in hospitalizations and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and should ask for medical advice when observing any indicative signs or symptoms. Patients should be advised to stop the drug immediately if they develop any type of rash or contact their physicians as soon as possible.
- Advise patients to be alert for the symptoms of congestive heart failure including shortness of breath, unexplained weight gain, or edema and to contact their healthcare provider if such symptoms occur (see WARNINGS).
- Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If these occur, patients should be instructed to stop therapy and seek immediate medical therapy.
- Patients should be informed of the signs of an anaphylactoid reaction (e.g., difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help (see WARNINGS).
- In late pregnancy, as with other NSAIDs, Ibuprofen Oral Suspension should be avoided because it may cause premature closure of the ductus arteriosus.
Laboratory Tests
Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. Patients on long-term treatment with NSAIDs should have their CBC and a chemistry profile checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash, etc.) or if abnormal liver tests persist or worsen, Ibuprofen Oral Suspension should be discontinued.
Drug Interactions
ACE-inhibitors
Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE-inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE-inhibitors.
Aspirin
As with other NSAIDs, concomitant administration of ibuprofen and aspirin is not generally recommended because of the potential of increased adverse effects.
Diuretics
Clinical studies, as well as post marketing observations, have shown that ibuprofen oral suspension can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure (see WARNINGS - Renal Effects), as well as to assure diuretic efficacy.
Lithium
Ibuprofen produced an elevation of plasma lithium levels and a reduction in renal lithium clearance in a study of eleven normal volunteers. The mean minimum lithium concentration increased 15% and the renal clearance of lithium was decreased by 19% during this period of concomitant drug administration. This effect has been attributed to inhibition of renal prostaglandin synthesis by ibuprofen. Thus, when ibuprofen and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity. (Read circulars for lithium preparation before use of such concurrent therapy.)
Methotrexate
NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.
Warfarin
Several short-term controlled studies failed to show that ibuprofen significantly affected prothrombin times or a variety of other clotting factors when administered to individuals on warfarin-type anticoagulants. However, because bleeding has been reported when ibuprofen and other NSAIDs have been administered to patients on warfarin-type anticoagulants, the physician should be cautious when administering ibuprofen to patients on anticoagulants. The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that the users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Reproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities. However, animal reproduction studies are not always predictive of human response. There are no adequate and well-controlled studies in pregnant women. Ibuprofen should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery
In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition, and decreased pup survival occurred. The effects of ibuprofen suspension on labor and delivery in pregnant women are unknown. Therefore, administration of Ibuprofen Oral Suspension is not recommended during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Ibuprofen Oral Suspension, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness of ibuprofen oral suspension in pediatric patients below the age of 6 months have not been established (see CLINICAL PHARMACOLOGY - Clinical Studies). Dosing of Ibuprofen Oral Suspension in children 6 months or older should be guided by their body weight (see DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
In patients taking ibuprofen or other NSAIDs, the most frequently reported adverse experiences occurring in approximately 1-10% of patients are: abnormal renal function, anemia, dizziness, edema, elevated liver enzymes, fluid retention, gastrointestinal experiences (including abdominal pain, bloating, constipation, diarrhea, dyspepsia, epigastric pain, flatulence, heartburn, nausea, vomiting), headaches, increased bleeding time, nervousness, pruritus, rashes (including maculopapular) and tinnitus.
Additional adverse experiences reported occasionally include:
Body as a whole - fever, infection, sepsis Cardiovascular system - congestive heart failure in patients with marginal cardiac function, hypertension, tachycardia, syncope Digestive system - dry mouth, duodenitis, esophagitis, gastric or duodenal ulcer with bleeding and/or perforation, gastritis, gastrointestinal bleeding, glossitis, hematemesis, hepatitis, jaundice, melena, rectal bleeding Hemic and lymphatic system - ecchymosis, eosinophilia, leukopenia, purpura, stomatitis, thrombocytopenia Metabolic and nutritional - weight changes Nervous system - anxiety, asthenia, confusion, depression, dream abnormalities, drowsiness, insomnia, malaise, paresthesia, somnolence, tremors, vertigo Respiratory system - asthma, dyspnea Skin and appendages - alopecia, photosensitivity, sweat Special senses - blurred vision Urogenital system - cystitis, dysuria, hematuria, interstitial nephritis, oliguria/polyuria, proteinuria, acute renal failure in patients with pre-existing significantly impaired renal function Other adverse reactions, which occur rarely are:
Body as a whole - anaphylactic reactions, anaphylactoid reactions, appetite changes Cardiovascular system - arrhythmia, cerebrovascular accident, hypotension, myocardial infarction, palpitations, vasculitis Digestive system - eructation, gingival ulcer, hepatorenal syndrome, liver necrosis, liver failure, pancreatitis Hemic and lymphatic system - agranulocytosis, hemolytic anemia, aplastic anemia, lymphadenopathy, neutropenia, pancytopenia Metabolic and nutritional - hyperglycemia Nervous system - convulsions, coma, emotional lability, hallucinations, aseptic meningitis Respiratory - apnea, respiratory depression, pneumonia, rhinitis Skin and appendages - angioedema, toxic epidermal necrosis, erythema multiforme, exfoliative dermatitis, Stevens Johnson syndrome, urticaria, vesiculobullous eruptions Special senses - amblyopia (blurred and/or diminished vision, scotomata and/or changes in color vision), conjunctivitis, dry eyes, hearing impairment Urogenital - azotemia, decreased creatinine clearance, glomerulitis, renal papillary necrosis, tubular necrosis -
OVERDOSAGE
The toxicity of ibuprofen overdose is dependent upon the amount of drug ingested and the time elapsed since ingestion, though individual response may vary, which makes it necessary to evaluate each case individually. Although uncommon, serious toxicity and death have been reported in the medical literature with ibuprofen overdosage. The most frequently reported symptoms of ibuprofen overdose include abdominal pain, nausea, vomiting, lethargy and drowsiness. Other central nervous system symptoms include headache, tinnitus, CNS depression and seizures. Metabolic acidosis, coma, acute renal failure and apnea (primarily in very young children) may rarely occur. Cardiovascular toxicity, including hypotension, bradycardia, tachycardia and atrial fibrillation, also have been reported.
The treatment of acute ibuprofen overdose is primarily supportive. Management of hypotension, acidosis and gastrointestinal bleeding may be necessary. In cases of acute overdose, the stomach should be emptied through ipecac-induced emesis or lavage. Emesis is most effective if initiated within 30 minutes of ingestion. Orally administered activated charcoal may help in reducing the absorption and reabsorption of ibuprofen.
In children, the estimated amount of ibuprofen ingested per body weight may be helpful to predict the potential for development of toxicity although each case must be evaluated. Ingestion of less than 100 mg/kg is unlikely to produce toxicity. Children ingesting 100 to 200 mg/kg may be managed with induced emesis and a minimal observation time of four hours. Children ingesting 200 to 400 mg/kg of ibuprofen should have immediate gastric emptying and at least four hours observation in a health care facility. Children ingesting greater than 400 mg/kg require immediate medical referral, careful observation and appropriate supportive therapy. Ipecac-induced emesis is not recommended in overdoses greater than 400 mg/kg because of the risk for convulsions and the potential for aspiration of gastric contents.
In adult patients the history of the dose reportedly ingested does not appear to be predictive of toxicity. The need for referral and follow-up must be judged by the circumstances at the time of the overdose ingestion. Symptomatic adults should be carefully evaluated, observed and supported.
-
DOSAGE AND ADMINISTRATION
Carefully consider the potential benefits and risks of Ibuprofen Oral Suspension and other treatment options before deciding to use Ibuprofen Oral Suspension. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).
After observing the response to initial therapy with Ibuprofen Oral Suspension, the dose and frequency should be adjusted to suit an individual patient's needs.
PEDIATRIC PATIENTS
Fever Reduction
For reduction of fever in children, 6 months up to 2 years of age, the dosage should be adjusted on the basis of the initial temperature level (see CLINICAL PHARMACOLOGY). The recommended dose is 5 mg/kg if the baseline temperature is less than 102.5°F, or 10 mg/kg if the baseline temperature is 102.5°F or greater. The duration of fever reduction is generally 6 to 8 hours. The recommended maximum daily dose is 40 mg/kg.
Analgesia
For relief of mild to moderate pain in children 6 months up to 2 years of age, the recommended dosage is 10 mg/kg, every 6 to 8 hours. The recommended maximum daily dose is 40 mg/kg. Doses should be given so as not to disturb the child's sleep pattern.
Juvenile Arthritis
The recommended dose is 30 to 40 mg/kg/day divided into three to four doses (see Individualization of Dosage). Patients with milder disease may be adequately treated with 20 mg/kg/day.
In patients with juvenile arthritis, doses above 50 mg/kg/day are not recommended because they have not been studied and doses exceeding the upper recommended dose of 40 mg/kg/day may increase the risk of causing serious adverse events. The therapeutic response may require from a few days to several weeks to be achieved. Once a clinical effect is obtained, the dosage should be lowered to the smallest dose of ibuprofen needed to maintain adequate control of symptoms.
Pediatric patients receiving doses above 30 mg/kg/day or if abnormal liver function tests have occurred with previous NSAID treatments should be carefully followed for signs and symptoms of early liver dysfunction.
ADULTS
Primary Dysmenorrhea
For the treatment of primary dysmenorrhea, beginning with the earliest onset of such pain, Ibuprofen Oral Suspension should be given in a dose of 400 mg every 4 hours, as necessary, for the relief of pain.
Rheumatoid Arthritis and Osteoarthritis
Suggested dosage: 1200-3200 mg daily (300 mg q.i.d. or 400 mg, 600 mg or 800 mg t.i.d. or q.i.d.). Individual patients may show a better response to 3200 mg daily, as compared with 2400 mg, although in well-controlled clinical trials patients on 3200 mg did not show a better mean response in terms of efficacy. Therefore, when treating patients with 3200 mg/day, the physician should observe sufficient increased clinical benefits to offset potential increased risk.
Individualization of Dosage
The dose of Ibuprofen Oral Suspension should be tailored to each patient, and may be lowered or raised from the suggested doses depending on the severity of symptoms either at time of initiating drug therapy or as the patient responds or fails to respond.
One fever study showed that, after the initial dose of ibuprofen oral suspension, subsequent doses may be lowered and still provide adequate fever control.
In a situation when low fever would require the ibuprofen oral suspension 5 mg/kg dose in a child with pain, the dose that will effectively treat the predominant symptom should be chosen.
In chronic conditions, a therapeutic response to ibuprofen oral suspension therapy is sometimes seen in a few days to a week, but most often is observed by two weeks. After a satisfactory response has been achieved, the patient's dose should be reviewed and adjusted as required.
Patients with rheumatoid arthritis seem to require higher doses than do patients with osteoarthritis. The smallest dose of Ibuprofen Oral Suspension that yields acceptable control should be employed.
Ibuprofen Oral Suspension may be used in combination with gold salts and/or corticosteroids.
-
HOW SUPPLIED
Ibuprofen Oral Suspension USP, 100 mg/5 mL is an orange-colored, berry-flavored suspension supplied in the following oral dosage forms:
NDC: 0121-4774-05: 5 mL unit dose cup NDC: 0121-4774-40: Case contains 40 unit dose cups of 5 mL (0121-4774-05) packaged in 4 trays of 10 unit dose cups each. NDC: 0121-1548-10: 10 mL unit dose cup NDC: 0121-1548-40: Case contains 40 unit dose cups of 10 mL (0121-1548-10) packaged in 4 trays of 10 unit dose cups each. - SPL UNCLASSIFIED SECTION
-
Medication Guide for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs (NSAIDs)?
NSAIDs can cause serious side effects, including:
-
Increased risk of a heart attack or stroke that can lead to death. This risk may happen early in treatment and may increase:
- with increasing doses of NSAIDs
- with longer use of NSAIDs
Do not take NSAIDs right before or after a heart surgery called a "coronary artery bypass graft (CABG)."
Avoid taking NSAIDs after a recent heart attack, unless your healthcare provider tells you to. You may have an increased risk of another heart attack if you take NSAIDs after a recent heart attack.
-
Increased risk of bleeding, ulcers, and tears (perforation) of the esophagus (tube leading from the mouth to the stomach), stomach and intestines:
- anytime during use
- without warning symptoms
- that may cause death
The risk of getting an ulcer or bleeding increases with:
- past history of stomach ulcers, or stomach or intestinal bleeding with use of NSAIDs
- taking medicines called "corticosteroids", "anticoagulants", "SSRIs", or "SNRIs"
- increasing doses of NSAIDs
- longer use of NSAIDs
- smoking
- drinking alcohol
- older age
- poor health
- advanced liver disease
- bleeding problems
NSAIDs should only be used:
- exactly as prescribed
- at the lowest dose possible for your treatment
- for the shortest time needed
What are NSAIDs?
NSAIDs are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as different types of arthritis, menstrual cramps and other types of short-term pain.
Who should not take NSAIDs?
Do not take NSAIDs:
- if you have had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAIDs
- right before or after heart bypass surgery.
Before taking NSAIDs, tell your healthcare provider about all of your medical conditions, including if you:
- have liver or kidney problems
- have high blood pressure
- have asthma
- are pregnant or plan to become pregnant. Talk to your healthcare provider if you are considering taking NSAIDs during pregnancy. You should not take NSAIDs after 29 weeks of pregnancy.
- are breastfeeding or plan to breast feed.
Tell your healthcare provider about all of the medicines you take, including prescription or over-the-counter medicines, vitamins or herbal supplements. NSAIDs and some other medicines can interact with each other and cause serious side effects. Do not start taking any new medicine without talking to your healthcare provider first.
What are the possible side effects of NSAIDs?
NSAIDs can cause serious side effects, including:
- new or worse high blood pressure
- heart failure
- liver problems including liver failure
- kidney problems including kidney failure
- low red blood cells (anemia)
- life-threatening skin reactions
- life-threatening allergic reactions
- Other side effects of NSAIDs include: stomach pain, constipation, diarrhea, gas, heartburn, nausea, vomiting, and dizziness.
Get emergency help right away if you have any of the following symptoms:
- shortness of breath or trouble breathing
- chest pain
- a weakness in one part or side of your body
- slurred speech
- swelling of the face or throat
Stop taking your NSAID and call your healthcare provider right away if you get any of the following symptoms:
- nausea
- more tired or weaker than usual
- diarrhea
- itching
- your skin or eyes look yellow
- indigestion or stomach pain
- flu-like symptoms
- vomit blood
- there is blood in your bowel movement or it is black and sticky like tar
- unusual weight gain
- skin rash or blisters with fever
- swelling of the arms, legs, hands and feet
If you take too much of your NSAID, call your healthcare provider or get medical help right away.
These are not all the possible side effects with NSAIDs. For more information, ask your healthcare provider or pharmacist about NSAIDs.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Other information about NSAIDs
- Aspirin is an NSAID but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
- Some NSAIDs are sold in lower doses without a prescription (over-the-counter). Talk to your healthcare provider before using over-the-counter NSAIDs for more than 10 days.
General information about the safe and effective use of NSAIDs
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NSAIDs for a condition for which it was not prescribed. Do not give NSAIDs to other people, even if they have the same symptoms that you have. It may harm them.
If you would like more information about NSAIDs, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about NSAIDs that is written for health professionals.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
MANUFACTURED BY
Perrigo Pharmaceuticals Company
Allegan, MI 49010PACKAGED BY
Pharmaceutical
Associates, Inc.
Greenville, SC 29605
www.paipharma.comR07/17
-
Increased risk of a heart attack or stroke that can lead to death. This risk may happen early in treatment and may increase:
-
PRINCIPAL DISPLAY PANEL - 5 mL Cup Label
Delivers 5 mL
NDC: 0121-4774-05I BUPROFEN
O RAL S USPENSION USP100 mg/5 mL SHAKE WELL
FOR INSTITUTIONAL USE ONLY
Rx ONLYMFG. BY: PERRIGO PHARMACEUTICALS CO.
ALLEGAN, MI 49010PKG. BY: PHARMACEUTICAL ASSOCIATES, INC.
GREENVILLE, SC 29605SEE INSERT
A47740500

-
PRINCIPAL DISPLAY PANEL - 10 mL Cup Label
Delivers 10 mL
NDC: 0121-1548-10I BUPROFEN
O RAL S USPENSION USP200 mg/10 mL
SHAKE WELLFOR INSTITUTIONAL USE ONLY
Rx ONLYPKG. BY: PHARMACEUTICAL ASSOCIATES, INC.
GREENVILLE, SC 29605SEE INSERT
A4774100917

-
INGREDIENTS AND APPEARANCE
IBUPROFEN
ibuprofen suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0121-4774(NDC:45802-952) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength BUTYLPARABEN (UNII: 3QPI1U3FV8) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color orange Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0121-4774-40 4 in 1 CASE 12/11/2017 1 10 in 1 TRAY 1 NDC: 0121-4774-05 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product 2 NDC: 0121-4774-10 4 in 1 CASE 12/11/2017 2 10 in 1 TRAY 2 10 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076925 09/23/2004 IBUPROFEN
ibuprofen suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0121-1548 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength BUTYLPARABEN (UNII: 3QPI1U3FV8) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color orange Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0121-1548-40 4 in 1 CASE 12/11/2017 1 10 in 1 TRAY 1 NDC: 0121-1548-10 10 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076925 09/23/2004 Labeler - Pharmaceutical Associates, Inc. (044940096) Establishment Name Address ID/FEI Business Operations L. Perrigo Company 006013346 manufacture(0121-4774, 0121-1548) Establishment Name Address ID/FEI Business Operations Pharmaceutical Associates, Inc. 097630693 repack(0121-4774, 0121-1548)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.