WAYRILZ- rilzabrutinib tablet, film coated
WAYRILZ by
Drug Labeling and Warnings
WAYRILZ by is a Prescription medication manufactured, distributed, or labeled by Genzyme Corporation, Patheon Inc (TRO), Sanofi S.r.l., Flamma S.p.A., Flamma SpA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use WAYRILZ™ safely and effectively. See full prescribing information for WAYRILZ.
WAYRILZ™ (rilzabrutinib) tablets, for oral use
Initial U.S. Approval: 2025INDICATIONS AND USAGE
WAYRILZ is a kinase inhibitor indicated for the treatment of adult patients with persistent or chronic immune thrombocytopenia (ITP) who have had an insufficient response to a previous treatment. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 400 mg (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Serious Infections: Monitor patients for signs and symptoms of infection, evaluate promptly, and treat. (5.1)
- Hepatotoxicity, Including Drug-Induced Liver Injury: Evaluate bilirubin and transaminases at baseline and as clinically indicated during treatment. (5.2)
- Embryo-Fetal Toxicity: Based on preliminary animal data, WAYRILZ may cause fetal harm. Advise females of reproductive potential of the potential risk and to use effective contraception. (5.3, 8.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥10%) were diarrhea, nausea, headache, abdominal pain, and COVID-19. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genzyme Corporation at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- CYP3A Inhibitors: Avoid co-administration with moderate or strong CYP3A inhibitors. (7.1)
- CYP3A Inducers: Avoid co-administration with moderate or strong CYP3A inducers. (7.1)
- Gastric Acid Reducing Agents: Avoid co-administration with proton pump inhibitors (PPIs). WAYRILZ should be administered at least 2 hours before taking an antacid or H2 receptor antagonist. (7.1)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Testing Before Initiating WAYRILZ
2.2 Recommended Dosage
2.3 Monitoring and Dose Modifications for Hepatotoxicity
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
5.2 Hepatotoxicity, Including Drug-Induced Liver Injury
5.3 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on WAYRILZ
7.2 Effect of WAYRILZ on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Testing Before Initiating WAYRILZ
Verify pregnancy status of females of reproductive potential prior to initiating WAYRILZ treatment [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1, 8.3)].
2.2 Recommended Dosage
The recommended dosage of WAYRILZ is 400 mg taken orally twice daily.
WAYRILZ can be taken at approximately the same time each day with or without food. In patients who experience gastrointestinal symptoms, taking WAYRILZ with food may improve tolerability. Advise patients to swallow tablets whole with a glass of water. Advise patients not to cut, crush or chew the tablets.
If a dose is missed, patients should take the missed dose of WAYRILZ as soon as possible on the same day and at least 2 hours apart from the next regular scheduled dose.
If taking antacid or histamine H2 receptor antagonist, administer the dose of WAYRILZ at least 2 hours before the antacid or histamine H2 receptor antagonist.
2.3 Monitoring and Dose Modifications for Hepatotoxicity
Evaluate bilirubin and transaminases at baseline and as clinically indicated during treatment with WAYRILZ. For patients who develop abnormal liver tests after WAYRILZ, monitor more frequently for liver test abnormalities and clinical signs and symptoms of hepatic toxicity. If Drug-Induced Liver Injury (DILI) is suspected, withhold WAYRILZ. Upon confirmation of DILI, discontinue WAYRILZ.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
An increased risk of serious infections (including bacterial, viral, or fungal) can occur in patients treated with Bruton's tyrosine kinase (BTK) inhibitors, including WAYRILZ. In the LUNA-3 trial, fatal pneumonia occurred in one participant in the WAYRILZ group. Other serious infections [one each (0.8%)] included COVID-19 infection, wound infection, and one patient experienced urinary tract infection and kidney abscess. Monitor patients for signs and symptoms of infection and treat appropriately.
5.2 Hepatotoxicity, Including Drug-Induced Liver Injury
Hepatotoxicity, including severe, life-threatening, and potentially fatal cases of DILI, can occur in patients treated with BTK inhibitors. In the clinical trials of WAYRILZ in patients with ITP, elevations of liver transaminases occurred and were generally mild to moderate in severity. Evaluate bilirubin and transaminases at baseline and as clinically indicated during treatment with WAYRILZ. For patients who develop abnormal liver tests after WAYRILZ, monitor more frequently for liver test abnormalities and clinical signs and symptoms of hepatic toxicity. If DILI is suspected, withhold WAYRILZ. Upon confirmation of DILI, discontinue WAYRILZ.
5.3 Embryo-Fetal Toxicity
Based on findings from preliminary animal reproduction studies, WAYRILZ may cause fetal harm when administered to a pregnant woman. Adverse visceral and skeletal findings occurred in rat fetuses at a maternally toxic dose at exposures 22-times the human exposure [based on area under the curve (AUC)] at the maximum recommended human dose (MRHD), and renal visceral malformations occurred in a single rabbit fetus at 5.6-times the human exposure (based on AUC) at the MRHD. Verify pregnancy status of females of reproductive potential prior to initiating WAYRILZ treatment. Advise females of reproductive potential to use an effective method of contraception during treatment with WAYRILZ and for 1 week after the final dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically important adverse reactions are described elsewhere in the labeling:
- Serious Infections [see Warnings and Precautions (5.1)]
- Hepatotoxicity, Including Drug-Induced Liver Injury [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of WAYRILZ was evaluated in a randomized, double-blind (DB), placebo-controlled, parallel-group study (LUNA-3), in which 202 adult patients with persistent or chronic ITP received either WAYRILZ (n=133) or placebo (n=69) [see Clinical Studies (14)].
During the 24-week DB period, the median duration of WAYRILZ exposure was 98 days (range: 22 to 182).
The most common adverse reactions (≥10%) were diarrhea, nausea, headache, abdominal pain, and COVID-19. Adverse reactions resulting in discontinuation of WAYRILZ included erythema nodosum, neutropenia, arthralgia, dyspepsia, headache, pain in extremity, abdominal discomfort, diarrhea, nausea, and pneumonia and occurred in 4.5% of patients.
Table 1 presents common adverse reactions from the LUNA-3 Study.
Table 1: Common Adverse Reactions* in Patients with ITP During Double-Blind Period of the LUNA-3 Study Adverse Reactions WAYRILZ
(N=133)Placebo
(N=69)All Grades
%Grade 3 or Higher
%All Grades
%Grade 3 or Higher
%- * Adverse reactions that occurred in at least 5% of WAYRILZ treated patients and at least 3% higher than placebo-treated patients.
- † Grouped term
Diarrhea 32 0 10 0 Nausea 20 0 6 0 Headache 18 0 7 0 Abdominal Pain† 14 0 1 0 COVID-19 14 0.8 4 0 Arthralgia 9 0 4 0 Dizziness 8 0 1 0 Nasopharyngitis† 7 0 3 0 Vomiting 7 0 1 0 Dyspepsia 5 0 0 0 Cough* 5 0 0 0 Specific Adverse Reactions
Gastrointestinal Events
In the LUNA-3 Study DB period, the most common gastrointestinal (GI) adverse reactions were diarrhea (32%), nausea (20%), and abdominal pain (14%) in the WAYRILZ group. These events were Grade 1 or 2. Of those who experienced GI adverse reactions, 2 patients discontinued due to GI adverse reactions. Recovery or resolution with supportive treatment allowing continuation of WAYRILZ treatment occurred in 91% of patients with diarrhea, 85% with nausea and 79% with abdominal pain.
Neutropenia
In the LUNA-3 Study DB period, Grade 1 or 2 neutropenia occurred in 11% of patients in the WAYRILZ group.
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on WAYRILZ
Strong and Moderate CYP3A Inhibitors
Avoid concomitant use of WAYRILZ with strong or moderate CYP3A inhibitors. If a strong or moderate CYP3A inhibitor cannot be avoided, and these inhibitors will be used short term (such as anti-infectives for seven days or less), interrupt treatment with WAYRILZ. Avoid concomitant use of grapefruit, starfruit and products containing these fruits, and Seville oranges with WAYRILZ, as these are moderate and strong inhibitors of CYP3A.
Rilzabrutinib is a CYP3A substrate. Concomitant use with a strong or moderate CYP3A inhibitor increases rilzabrutinib Cmax and AUC [see Clinical Pharmacology (12.3)], which increases the risk of WAYRILZ adverse reactions.
Strong and Moderate CYP3A Inducers
Avoid concomitant use of WAYRILZ with strong or moderate CYP3A inducers.
Rilzabrutinib is a CYP3A substrate. Concomitant use with a strong or moderate CYP3A inducer decreases rilzabrutinib Cmax and AUC [see Clinical Pharmacology (12.3)], which may reduce WAYRILZ efficacy.
Gastric Acid Reducing Agents
Administer the dose of WAYRILZ at least 2 hours before administration of an antacid or histamine H2 receptor antagonist. Avoid concomitant use of proton pump inhibitors with WAYRILZ.
Rilzabrutinib exhibits pH-dependent solubility. Acid reducing agents decrease rilzabrutinib exposure [see Clinical Pharmacology (12.3)], which may reduce WAYRILZ efficacy.
7.2 Effect of WAYRILZ on Other Drugs
CYP3A Substrates
Monitor for adverse reactions of the concurrently administered CYP3A substrate more frequently and consider dosage adjustment in accordance with the Prescribing Information of the CYP3A substrate.
Rilzabrutinib is a moderate inhibitor of CYP3A and increases exposure of these substrates [see Clinical Pharmacology (12.3)], which increases the risk of adverse reactions related to these substrates.
P-gp, BCRP, and OATP1B Substrates
Monitor for adverse reactions of the concurrently administered P-gp, BCRP, or OATP1B substrate more frequently, unless otherwise recommended in the substrate Prescribing Information, when WAYRILZ is used concomitantly with P-gp, BCRP, or OATP1B substrates where minimal substrate concentration changes may lead to serious adverse reactions.
Rilzabrutinib is an inhibitor of P-gp, BCRP and OATP1B in vitro. The effect of concomitant use of WAYRILZ with OATP1B and BCRP substrates has not been established in clinical studies. However, based on in vitro inhibitory potential [see Clinical Pharmacology (12.3)], concomitant use of WAYRILZ may increase the risk of adverse reactions related to these substrates.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal data, WAYRILZ may cause fetal harm when administered to a pregnant woman. In animal reproduction studies, oral administration of rilzabrutinib to pregnant rats and rabbits during organogenesis at exposures 4- to 10-times the human exposure (based on AUC) at the maximum recommended human dose (MRHD) of 400 mg twice daily did not cause adverse developmental effects. However, adverse visceral and skeletal findings occurred in rat fetuses at a maternally toxic dose at exposures 22-times the human exposure (based on AUC) at the MRHD (see Data). There are no available clinical data on the use of WAYRILZ during pregnancy to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Advise pregnant women of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, and other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Animal Data
Rilzabrutinib given to pregnant rats by oral gavage at 50, 150 or 300 mg/kg/day during the period of organogenesis (gestation day 7 to 17) did not cause adverse effects on embryo-fetal development at exposures approximately 10-times the clinical exposure at the maximum recommended human dose (MRHD), based on AUC. Increased incidence of post-implantation loss (25%), delayed ossification associated with a markedly lower (24%) mean fetal weight, and increased skeletal (scoliosis) and visceral malformations (abnormalities of major vessels, urogenital tract, and kidney) occurred in a preliminary study in rats at a maternally toxic dose of 500 mg/kg/day that resulted in exposures 22-times the clinical exposure at the MRHD, based on AUC.
Rilzabrutinib given to pregnant rabbits by oral gavage at 10, 30 or 100 mg/kg/day during the period of organogenesis (gestation day 7 to 19) did not cause adverse effects on embryo-fetal development at exposures approximately 4-times the clinical exposures at the MRHD, based on AUC. Renal visceral malformations occurred in a single fetus in a preliminary study in rabbits at 150 mg/kg/day that resulted in exposures 5.6-times the clinical exposure at the MRHD, based on AUC.
In a pre- and postnatal developmental toxicity study, pregnant rats were given rilzabrutinib by oral gavage at doses of 50, 150 or 300 mg/kg/day during the periods of gestation, parturition, and lactation (gestation day 7 to lactation day 21). Decreased body weight was observed in pups in the presence of maternal toxicity at 300 mg/kg/day, at exposures approximately 18-times the MRHD based on AUC. There were no effects of rilzabrutinib on the ability of F1 offspring to reproduce or on subsequent F2 development at any dose.
8.2 Lactation
Risk Summary
There are no data on the presence of rilzabrutinib or its metabolites in either human or animal milk, the effects on the breastfed child, or the effects on milk production. Due to the potential for adverse reactions in a breastfed child from WAYRILZ, advise lactating women not to breastfeed while taking WAYRILZ and for at least 1 week after the final dose.
8.3 Females and Males of Reproductive Potential
Based on preliminary animal data, WAYRILZ may cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status of females of reproductive potential prior to initiating WAYRILZ treatment.
Contraception
Females
Advise female patients of reproductive potential to use effective contraception during WAYRILZ treatment and for 1 week after stopping treatment.
8.4 Pediatric Use
Safety and effectiveness of WAYRILZ have not been established in pediatric patients.
8.5 Geriatric Use
Of the 202 patients in the LUNA-3 study [see Clinical Studies (14)], 36 (18%) patients were 65 years of age and older, and 9 (5%) patients were 75 years of age and older. No overall differences in safety and efficacy were observed between patients 65 years of age and older and younger adult patients.
8.6 Hepatic Impairment
Avoid administration of WAYRILZ in patients with moderate or severe hepatic impairment (Child-Pugh class B-C) because of potential for increased rilzabrutinib exposures. Dose modification is not required for patients with mild hepatic impairment (Child-Pugh class A) [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
Avoid use in patients with severe renal impairment. Patients with severe renal impairment were not studied. Dose modification is not required for patients with mild or moderate renal impairment [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
WAYRILZ (rilzabrutinib) is a kinase inhibitor. Rilzabrutinib is a white to off-white solid, which is freely soluble in ethanol, sparingly soluble in isopropyl alcohol and practically insoluble in water.
The chemical name for rilzabrutinib is 1-Piperidinepropanenitrile, 3-[4-amino-3-(2-fluoro-4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-α-[2-methyl-2-[4-(3-oxetanyl)-1-piperazinyl]propylidene]-β-oxo-, (3R)-. The molecular formula is C36H40FN9O3 and the molecular weight is 665.77 Daltons. The chemical structural formula of rilzabrutinib is shown below:
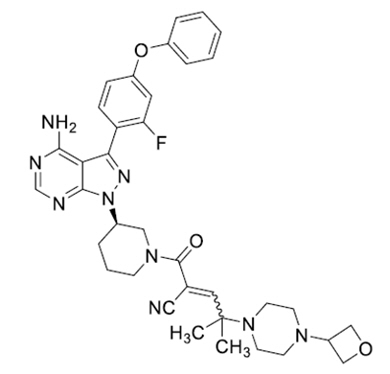
Each WAYRILZ film-coated tablet for oral administration contains 400 mg rilzabrutinib. The inactive ingredients in the tablet core are crospovidone (Type A), microcrystalline cellulose, and sodium stearyl fumarate. The inactive ingredients in the tablet coating are FD&C yellow #6/Sunset yellow FCF aluminum lake, macrogol/polyethylene glycol (PEG), polyvinyl alcohol partially hydrolyzed, talc, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Rilzabrutinib is a small-molecule, covalent, reversible kinase inhibitor targeting Bruton's tyrosine kinase (BTK). Rilzabrutinib mediates its therapeutic effect in ITP through immune modulation including 1) inhibition of B cell activation, and 2) interruption of antibody-coated cell phagocytosis by Fcγ receptor (FcγR) in spleen and liver. In vitro, rilzabrutinib reduced autoantibody signaling mediated through the FcγR pathway, blocked B cell signaling, and decreased autoantibody generation through effects on B cell activation.
12.2 Pharmacodynamics
Plasma Exposure and BTK Occupancy
WAYRILZ has a short duration of systemic exposure with a long duration of action on the target due to its slow dissociation from BTK. At therapeutic doses in healthy participants, durable BTK occupancy in peripheral blood mononuclear cells was observed over a 24-hour period.
Cardiac Electrophysiology
In a Thorough QT study, concentration dependent shortening in the QTc interval was observed. Following a single dose of rilzabrutinib 400 mg, the mean maximum QTcF decrease of -7 msec (90% confidence interval: -9 msec to -5 msec) was observed at 2 hours post-dose. The mean maximum QTcF decrease at exposures 4-fold the highest recommended dose, i.e., 400 mg BID, was -10 msec (90% confidence interval: -12 msec to -8 msec) at 2 hours post-dose.
12.3 Pharmacokinetics
The pharmacokinetics of rilzabrutinib are presented as geometric mean (% coefficient of variation) unless otherwise specified. The Cmax and AUC of rilzabrutinib increase proportionally following administration of multiple doses of 300 mg to 600 mg. Steady-state plasma levels are reached within 3 days with accumulation up to 1.3-fold at the approved recommended dosage. Following daily doses of 400 mg rilzabrutinib twice daily, the steady-state Cmax and AUC24h are 150 ng/mL (56%) and 1540 ng.h/mL (57.5%), respectively.
Absorption
The absolute oral bioavailability of rilzabrutinib is 4.7%. Following a single oral dose of 400 mg rilzabrutinib, the median time to peak plasma concentration (Tmax) is approximately 2 hours.
Effect of Food:
Rilzabrutinib AUC and Cmax decreased by 20% and 31%, respectively, following administration of a single oral 400 mg dose with a high fat meal (approximately 1,000 calories with 50% of total caloric content from fat).
Distribution
The volume of distribution at terminal phase (Vz) after IV administration is 149L. Rilzabrutinib is 97.5% bound to plasma proteins and the blood-to-plasma ratio is 0.786.
Metabolism
Rilzabrutinib is predominantly metabolized by cytochrome P450 3A.
Elimination
The clearance of rilzabrutinib is time-independent. Following multiple doses of 400 mg twice daily rilzabrutinib in patients with ITP, mean CL/F ranged from 246 to 911 L/h. Based on the population pharmacokinetic analysis in patients with ITP, the mean CL/F was 516 L/h.
In Phase 1 studies, the half-life of rilzabrutinib ranged between 1.6 to 4.5 hours.
Excretion
Following administration of a single 400 mg 14C-labeled rilzabrutinib dose in healthy subjects, approximately 86% of the dose was recovered in feces (9% unchanged) and to a lesser extent in urine (~5%) and bile (~6%). Approximately 0.03% of rilzabrutinib was excreted unchanged in the urine.
Special Populations
No clinically significant differences in the pharmacokinetics of rilzabrutinib were observed based on age (12 to 80 years), sex, race, mild to moderate renal impairment (CLCR 46 to 89 mL/min), or mild hepatic impairment (Child-Pugh class A). Rilzabrutinib exposure (both Cmax and AUC) increased by approximately 4.5-fold in participants with moderate hepatic impairment (Child-Pugh class B). Patients with severe hepatic impairment (Child-Pugh class C) and CLCR <46 mL/min have not been studied.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Effect of Other Drugs on Rilzabrutinib
Strong CYP3A inhibitors: Concomitant use with ritonavir (strong CYP3A inhibitor) increased rilzabrutinib Cmax by approximately 5-fold and AUC by 8-fold at steady state.
Moderate CYP3A inhibitors: Concomitant use with moderate CYP3A inhibitors (fluconazole, erythromycin and verapamil) is predicted to increase rilzabrutinib Cmax and AUC by approximately 3-fold at steady state.
Weak CYP3A inhibitors: Cimetidine is predicted to increase rilzabrutinib Cmax by approximately 2-fold and AUC by 1.6-fold.
Strong CYP3A inducers: Coadministration of rifampin (strong CYP3A inducer) decreased rilzabrutinib Cmax and AUC by approximately 80% at steady state.
Moderate CYP3A inducers: Coadministration of moderate CYP3A inducers (efavirenz, rifabutin) is predicted to reduce rilzabrutinib Cmax and AUC by up to 70% at steady state.
Weak CYP3A inducers: Modafinil is predicted to reduce rilzabrutinib Cmax and AUC by 20%.
Proton pump inhibitor: Coadministration of esomeprazole (proton pump inhibitor) decreased rilzabrutinib Cmax by 55% and AUC by 51%.
H2 receptor antagonist: Administration of famotidine (H2 receptor antagonist) two hours after the evening dose of rilzabrutinib decreased the next morning dose of rilzabrutinib Cmax by 35% and AUC by 28%.
P-glycoprotein (P-gp) inhibitor: Coadministration of quinidine (P-gp inhibitor) increased rilzabrutinib Cmax and AUC by approximately 13% at steady state.
Effect of Rilzabrutinib on Other Drugs
CYP3A4 substrates: Concomitant use of a single dose of rilzabrutinib 400 mg with midazolam (sensitive CYP3A inhibitor) increased midazolam Cmax and AUC by 2.2-fold as observed in study with healthy participants. Midazolam AUC is predicted to increase up to 3.0-fold in patients with immune thrombocytopenia following coadministration with 400 mg rilzabrutinib twice daily.
In Vitro Studies
CYP Enzymes: Rilzabrutinib is a substrate of CYP3A. Rilzabrutinib is both an inhibitor and inducer of CYP3A.
Transporters: Rilzabrutinib is a substrate of P-gp and BCRP, but not a substrate for OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, or BSEP. Rilzabrutinib inhibits P-gp, BCRP, OATP1B1, OATP1B3, and BSEP at clinically relevant concentrations.
-
13 NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Rilzabrutinib was not carcinogenic in a 6-month study in Tg.rasH2 mice at doses up to 300 mg/kg/day. In a 2-year rat carcinogenicity study at doses of 10, 30 or 100 and 5, 15 or 50 mg/kg/day in males and females, respectively, there were no rilzabrutinib-related tumors. The non-carcinogenic dose was the highest dose tested of 100 mg/kg/day for males and 50 mg/kg/day for females.
Rilzabrutinib was not mutagenic in an in vitro bacterial reverse mutagenicity (Ames) assay, was not clastogenic in an in vitro human peripheral lymphocyte chromosomal aberration assay, nor was it clastogenic in an in vivo bone marrow micronucleus assay in rats.
In a fertility study in male and female rats, rilzabrutinib was administered for a minimum of 28 days (males) and 15 days (females) prior to cohabitation and continued through gestation day 7 at doses of 50, 150 or 300 mg/kg/day by oral gavage. Rilzabrutinib had no effect on mating, estrous cycle, fertility, sperm parameters, or any ovarian and uterine parameters at any dose.
-
14 CLINICAL STUDIES
Immune Thrombocytopenia (ITP)
The safety and efficacy of WAYRILZ in adult patients with primary persistent or chronic ITP was evaluated in a randomized, double-blind (DB), placebo-controlled, parallel-group study consisting of 24 weeks of blinded treatment followed by an open-label (OL) period [LUNA-3 Study (NCT04562766)]. Patients received an initial 12 weeks of DB period treatment. Those who achieved platelet count response at 12 weeks were eligible to continue the full 24-week DB period. The patients enrolled in this study had an unsustained response to either intravenous immunoglobulin (IVIg/anti-D) or corticosteroid (CS) or had a documented intolerance or insufficient response to any appropriate course of standard-of-care ITP therapy.
Patients were randomized 2:1 to receive WAYRILZ 400 mg or placebo twice daily and randomization was stratified based on prior splenectomy (yes/no) and severity of thrombocytopenia (platelet count <15 ×109/L or ≥15 ×109/L).
Concomitant ITP medicines [oral CS and/or thrombopoietin receptor agonist (TPO-RA)] were allowed at stable doses at least 2 weeks before the start of the study and throughout the DB period.
In the LUNA-3 Study, 202 patients were randomized and treated, 133 to the WAYRILZ group and 69 to the placebo group. At baseline, the median age was 47 years (range: 18 to 80 years), 63% were female, 62% were Caucasian, 32% Asian, 1% were Black or African American, 2% were American Indian or Alaska native, 20% were Hispanic or Latino/a, and 77% were not Hispanic or Latino/a. Baseline demographics were generally similar across groups with the exception of sex which was 59% female in the WAYRILZ group and 71% in the placebo group.
At baseline 93% of patients had chronic ITP (i.e., for 1 year or longer), with a median time since ITP diagnosis of 7.69 years (range: 0.3, 52.2), and 28% had undergone splenectomy. The median platelet count was 15.3 × 109/L, with almost half (48%) less than 15 × 109/L. The median number of prior therapies, including splenectomy, was 4 (range: 1, 15). Prior ITP treatments varied, with the most common prior therapies being CS (96%), TPO-RAs (69%), IVIg or anti-D immunoglobulins (55%), and anti-CD20 monoclonal antibody/rituximab (35%). In addition, at baseline 66% of patients received both CS and TPO-RAs. Baseline disease characteristics were generally similar across both groups.
During the DB period, the median duration of exposure was 98 days (range: 22 to 182) and 84 days (range: 17 to 173) for the WAYRILZ group and placebo group, respectively. The cumulative duration of treatment exposure was 44 participant-years and 18 participant-years for the WAYRILZ and placebo groups, respectively. Concomitant use of CS and/or TPO-RA with study drug occurred in 60% and 67% of patients in the WAYRILZ and placebo arms, respectively.
During the first 12 weeks of the DB period, 85 (63.9%) patients and 22 (31.9%) patients in the WAYRILZ group and placebo group, respectively, achieved platelet count response (≥50 × 109/L or between 30 × 109/L and <50 × 109/L and doubled from baseline). Those who achieved platelet count response were eligible to continue the DB period.
The efficacy of WAYRILZ was based on durable platelet response. A durable platelet response was defined as a weekly platelet count ≥50 × 109/L for ≥ two-thirds of at least 8 non-missing weekly scheduled platelet measurements during the last 12 weeks of the 24-week DB period in the absence of rescue therapy, provided that at least 2 non-missing weekly scheduled platelet measurements were ≥50 × 109/L during the last 6 weeks of the DB period.
The results of the major efficacy endpoints during the DB period are summarized in Table 2.
Table 2: LUNA-3 Study Outcomes During the 24-week DB Period – Adult Population Study Outcomes Statistic WAYRILZ
400 mg BID
(N=133)Placebo
(N=69)Abbreviations: LS=Least Square; NR=Not Reached; CI=Confidence Interval; SE=Standard Error. - * p-value was derived by Cochran-Mantel-Haenszel (CMH) test adjusted by stratification factors.
- † Numbers of weeks with platelet response was based on 24-week blinded treatment period. Platelet counts assessed within 4 weeks of rescue medication intake are considered as no response; missing weekly platelet counts due to any reasons are considered as no response. p-value was derived by a mixed-effect model with repeated measures on longitudinal binary data with treatment, stratification factors, week (Weeks 2 to 25), treatment-by-week interaction as categorical fix effects.
- ‡ Platelet count ≥50 × 109/L or between 30 × 109/L and <50 × 109/L and at least doubled from baseline in absence of rescue therapy; p-value was derived by log-rank test adjusted by stratification factors.
Durable Platelet Response* n (%) 31 (23.3) 0 (0) 95% CI 16.12, 30.49 0.00, 0.00 Risk difference (95% CI) vs placebo 23.1 (15.95, 30.31) p-value <0.0001 Number of Weeks with Platelet Response† ≥50 × 109/L or between 30 × 109/L and <50 × 109/L and doubled from baseline LS Mean (SE) 7.18 (0.75) 0.72 (0.35) LS Mean difference (SE) vs placebo 6.46 (0.78) 95% CI 4.92, 7.99 p-value <0.0001 ≥30 × 109/L and doubled from baseline LS Mean (SE) 6.95 (0.75) 0.64 (0.34) LS Mean difference (SE) vs placebo 6.31 (0.78) 95% CI 4.79, 7.83 p-value <0.0001 Time to First Platelet Response‡ Median number of days to first platelet count (95% CI) 36 (22, 44) NR Hazard ratio (95% CI) vs placebo 3.10 (1.95, 4.93) p-value <0.0001 Rescue medication was required by 33% and 58% of patients receiving WAYRILZ and placebo, respectively. The median time to first use of rescue therapy was not reached in the WAYRILZ group and 56 days in the placebo group.
During the OL period, 7/73 (10%) patients who received WAYRILZ during the DB period achieved a durable response for the first time.
-
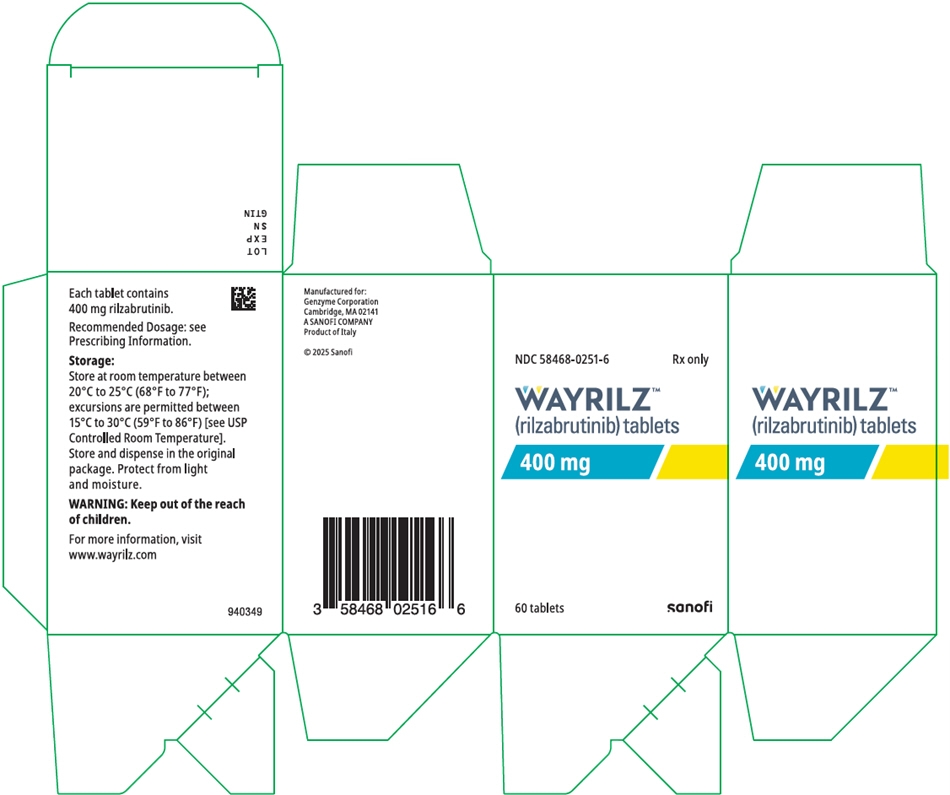
16 HOW SUPPLIED/STORAGE AND HANDLING
The 400 mg WAYRILZ film-coated tablets are orange, capsule-shaped, and debossed with "P" on one side and "400" on the other side.
How Supplied:
Package Size/Type Content NDC Number 60-count bottle Bottle containing 60 film-coated tablets with a child-resistant closure 58468-0251-6 56-count carton Carton containing 2 blister packs. Each blister pack (58468-0251-0) contains 28 film-coated tablets. 58468-0251-5 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Storage Instructions
Instruct patients to store WAYRILZ at room temperature in the original package and to protect from light and moisture.
Administration Instructions
Instruct patients to take WAYRILZ orally twice daily at approximately the same time each day with or without food. Advise patients that WAYRILZ tablets should be swallowed whole with a glass of water, and not to cut, crush or chew the tablets [see Dosage and Administration (2.2)].
Missed Dose
Advise patients that if they miss a dose of WAYRILZ, they should take it as soon as possible on the same day and at least 2 hours apart from the next regular scheduled dose.
Drug Interactions
Advise patients to inform their healthcare providers of all concomitant medications, including over-the-counter medications, vitamins, and herbal supplements. Advise patients to avoid eating grapefruit, starfruit, and Seville oranges and products containing these fruits with WAYRILZ [see Drug Interactions (7.1, 7.2)].
Serious Infections
Advise patients of the possibility of serious infection, and to report any signs or symptoms [see Warnings and Precautions (5.1)].
Hepatotoxicity, Including Drug-Induced Liver Injury
Inform patients that liver problems, including severe, life-threatening, or fatal hepatitis, DILI and abnormalities in liver tests, have occurred in patients treated with BTK inhibitors. Advise patients to contact their healthcare provider immediately if they experience abdominal discomfort, dark urine, or jaundice [see Warnings and Precautions (5.2)].
Embryo-Fetal Toxicity
Advise female patients of reproductive potential that WAYRILZ may cause fetal harm and to inform their healthcare providers of a known or suspected pregnancy. Advise females of reproductive potential to use effective contraception during treatment with WAYRILZ and for 1 week after the last dose [see Warnings and Precautions (5.3), Use in Specific Populations (8.1, 8.3)].
Lactation
Advise women not to breastfeed during treatment with WAYRILZ and for at least 1 week after the final dose [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
WAYRILZ™ (WAY-rilz)
(rilzabrutinib)
tablets, for oral useThis Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 08/2025 What is WAYRILZ?
WAYRILZ is a prescription medicine that is used to treat adults with persistent or chronic immune thrombocytopenia (ITP) who have received a prior treatment that did not work well enough.
It is not known if WAYRILZ is safe and effective in children.Before taking WAYRILZ, tell your healthcare provider about all of your medical conditions, including if you: - have liver problems
- have kidney problems
- are pregnant or plan to become pregnant. WAYRILZ may harm your unborn baby. If you are able to have a baby, your healthcare provider will do a pregnancy test before starting treatment with WAYRILZ.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with WAYRILZ and for 1 week after the last dose.
- are breastfeeding or plan to breastfeed. It is not known if WAYRILZ passes into your breast milk. Do not breastfeed during treatment with WAYRILZ and for at least 1 week after the last dose.
How should I take WAYRILZ? - Take WAYRILZ exactly as your healthcare provider tells you to take it.
- Do not change your dose or stop taking WAYRILZ unless your healthcare provider tells you to.
- Take WAYRILZ 2 times a day at about the same time each day.
- Take WAYRILZ with or without food.
- Swallow WAYRILZ tablets whole with a glass of water. Do not cut, crush, or chew the tablets.
- If you experience diarrhea, nausea, or stomach area (abdominal) pain during treatment with WAYRILZ, taking it with food may reduce these side effects.
- If you take an antacid or H2 blocker medicine, take your dose of WAYRILZ at least 2 hours before taking an antacid or H2 blocker medicine. If you take a Proton Pump Inhibitor (PPI) medicine, talk to your healthcare provider.
- If you miss a dose of WAYRILZ, take the missed dose as soon as you remember on the same day and at least 2 hours apart from the next regular scheduled dose.
- If you take too much WAYRILZ, call your healthcare provider or Poison Help Line at 1-800-222-1222.
What should I avoid while taking WAYRILZ? - You should avoid grapefruit, starfruit and products that have these fruits, and Seville oranges (often used in marmalades) during treatment with WAYRILZ. These products may increase the amount of WAYRILZ in your blood, which increases the risk of side effects of WAYRILZ.
What are the possible side effects of WAYRILZ?
WAYRILZ may cause serious side effects, including:- Serious infections. WAYRILZ can increase the risk of infections, including serious infections that can lead to death. Your healthcare provider will check you for signs and symptoms of infection during your treatment with WAYRILZ. Tell your healthcare provider right away if you get any signs or symptoms of infection, including fever, chills, or flu-like symptoms.
- Liver problems including Drug-Induced Liver Injury (DILI). Liver problems, which may be severe, life-threatening, or lead to death have happened in people treated with Bruton’s tyrosine kinase (BTK) inhibitors. Your healthcare provider will do blood tests to check your liver before and as necessary during treatment with WAYRILZ. Tell your healthcare provider right away if you have any signs or symptoms of liver problems, including stomach-area (abdominal) pain or discomfort, dark or “tea-colored” urine, or yellowing of the skin or the white part of your eyes.
- diarrhea
- nausea
- headache
- stomach area (abdominal) pain
- COVID-19
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store WAYRILZ? - Store WAYRILZ at room temperature between 20°C to 25°C (68°F to 77°F).
- Store WAYRILZ tablets in the original packaging.
- Protect WAYRILZ from light and moisture.
General information about the safe and effective use of WAYRILZ.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use WAYRILZ for a condition for which it was not prescribed. Do not give WAYRILZ to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about WAYRILZ that is written for health professionals.What are the ingredients in WAYRILZ?
Active ingredients: rilzabrutinib
Inactive ingredients: Tablet core: crospovidone (Type A), microcrystalline cellulose, and sodium stearyl fumarate; Tablet coating: FD&C yellow #6/Sunset yellow FCF aluminum lake, macrogol/polyethylene glycol (PEG), polyvinyl alcohol partially hydrolyzed, talc, and titanium dioxide.Manufactured for:
Genzyme Corporation
Cambridge, MA 02141
A SANOFI COMPANY
WAYRILZ is a trademark of Sanofi or an affiliate.
For more information go to www.wayrilz.com or call 1-833-723-5463. - PRINCIPAL DISPLAY PANEL - 60 Tablet Bottle Carton
-
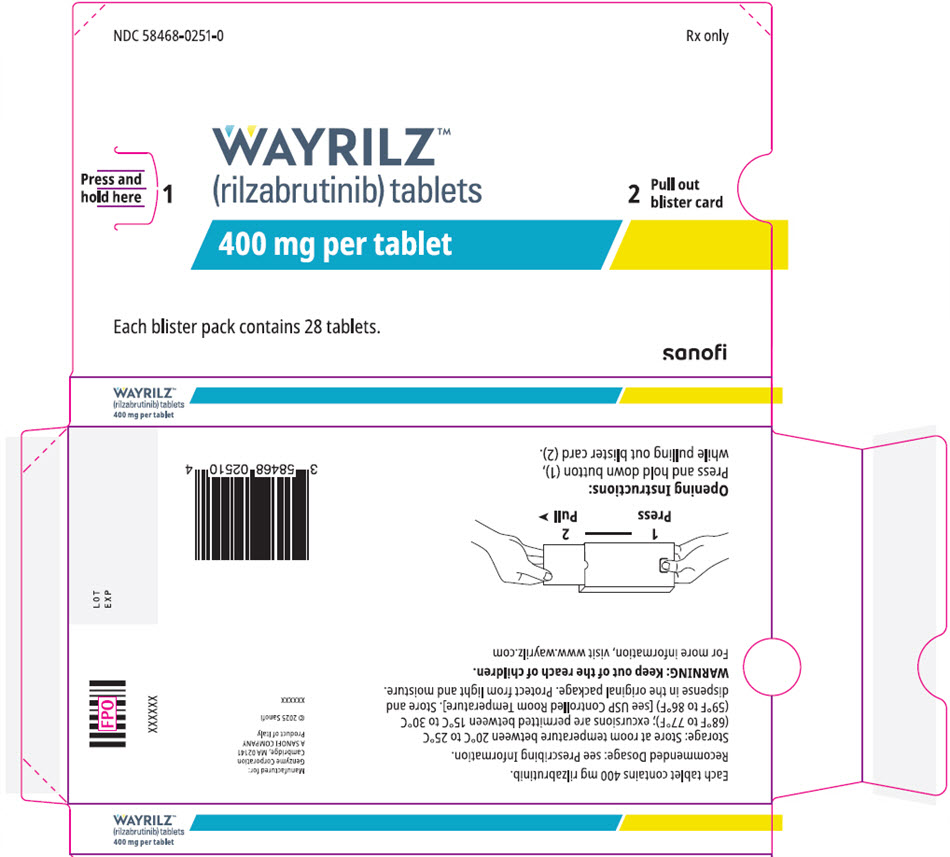
PRINCIPAL DISPLAY PANEL - 28 Tablet Blister Pack
NDC: 58468-0251-0
Rx onlyPress and
hold here
1WAYRILZ™
(rilzabrutinib) tablets
400 mg per tablet2
Pull out
blister cardEach blister pack contains 28 tablets.
sanofi
-
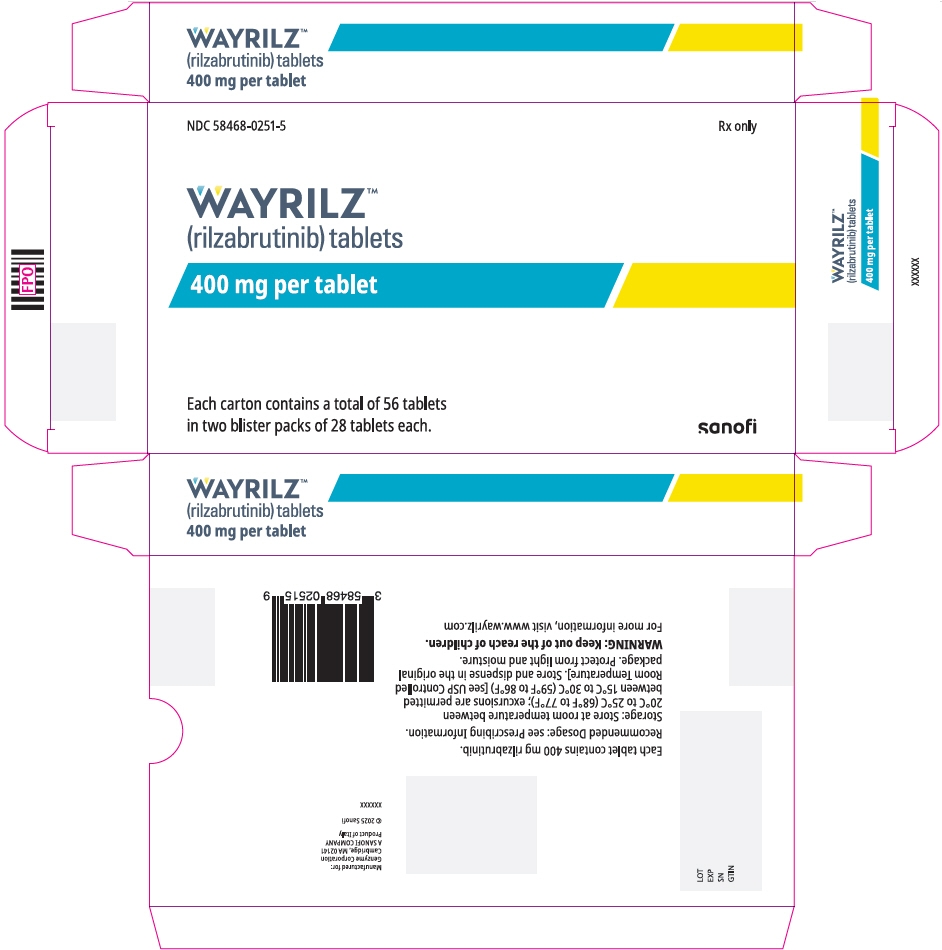
PRINCIPAL DISPLAY PANEL - 56 Tablet Blister Pack Carton
NDC: 58468-0251-5
Rx onlyWAYRILZ™
(rilzabrutinib) tablets
400 mg per tabletEach carton contains a total of 56 tablets
in two blister packs of 28 tablets each.sanofi
-
INGREDIENTS AND APPEARANCE
WAYRILZ
rilzabrutinib tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 58468-0251 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RILZABRUTINIB (UNII: NWN58M4F5T) (RILZABRUTINIB - UNII:NWN58M4F5T) RILZABRUTINIB 400 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) Product Characteristics Color ORANGE Score no score Shape OVAL (capsule-shaped) Size 17mm Flavor Imprint Code P;400 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58468-0251-6 1 in 1 CARTON 08/29/2025 1 60 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 58468-0251-5 2 in 1 CARTON 08/29/2025 2 NDC: 58468-0251-0 28 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA219685 08/29/2025 Labeler - Genzyme Corporation (025322157) Establishment Name Address ID/FEI Business Operations Patheon Inc (TRO) 240769596 ANALYSIS(58468-0251) , MANUFACTURE(58468-0251) , PACK(58468-0251) , LABEL(58468-0251) Establishment Name Address ID/FEI Business Operations Sanofi S.r.l. 543209212 ANALYSIS(58468-0251) , MANUFACTURE(58468-0251) , PACK(58468-0251) , LABEL(58468-0251) Establishment Name Address ID/FEI Business Operations Genzyme Corporation 050424395 ANALYSIS(58468-0251) , PACK(58468-0251) , LABEL(58468-0251) Establishment Name Address ID/FEI Business Operations Flamma S.p.A. 339093019 API MANUFACTURE(58468-0251) Establishment Name Address ID/FEI Business Operations Flamma SpA 339922561 API MANUFACTURE(58468-0251)
Trademark Results [WAYRILZ]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 WAYRILZ 98787964 not registered Live/Pending |
Principia Biopharma Inc. 2024-10-07 |
 WAYRILZ 90786128 not registered Live/Pending |
Principia Biopharma Inc. 2021-06-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.