LIDOCAINE PAIN RELIEF GEL PATCH- lidocaine pain relief patch
Lidocaine Pain Relief Gel Patch by
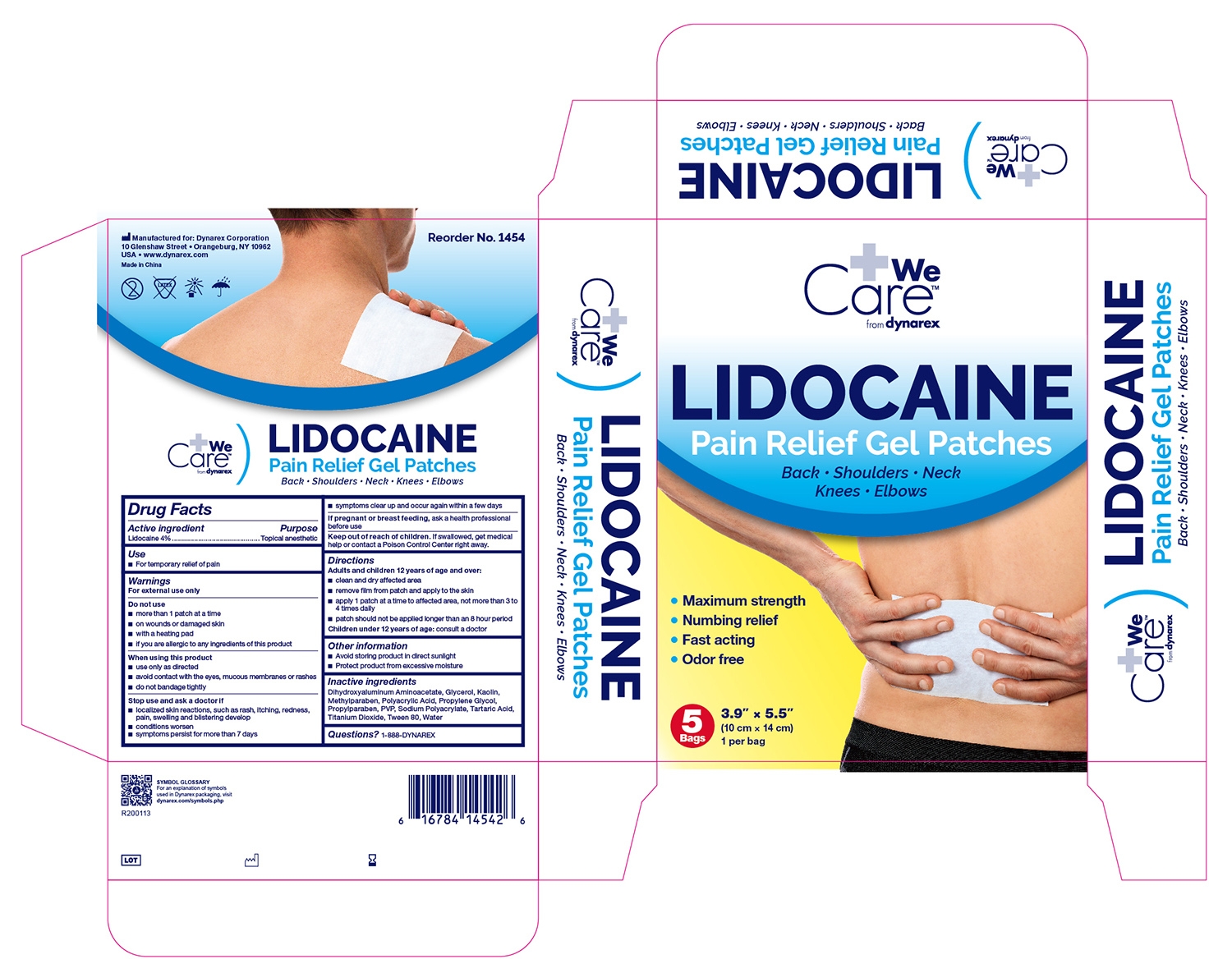
Drug Labeling and Warnings
Lidocaine Pain Relief Gel Patch by is a Otc medication manufactured, distributed, or labeled by Dynarex. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Puropose
- Use
-
Warnings
For external use only
Do not use
- more than 1 patch at a time
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any ingredients of this product
When using this product
- use only as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- Label
-
INGREDIENTS AND APPEARANCE
LIDOCAINE PAIN RELIEF GEL PATCH
lidocaine pain relief patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 67777-009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 40 mg in 1000 mg Inactive Ingredients Ingredient Name Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) METHYLPARABEN (UNII: A2I8C7HI9T) POVIDONE (UNII: FZ989GH94E) POLYACRYLIC ACID (8000 MW) (UNII: 73861X4K5F) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) TARTARIC ACID (UNII: W4888I119H) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) KAOLIN (UNII: 24H4NWX5CO) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67777-009-40 60 in 1 CASE 08/27/2019 1 5 in 1 BOX 1 40 mg in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 08/27/2019 Labeler - Dynarex (008124539) Registrant - Dynarex (008124539)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.