innisfree Daily UV Defense Sunscreen
innisfree Daily UV Defense Sunscreen by
Drug Labeling and Warnings
innisfree Daily UV Defense Sunscreen by is a Otc medication manufactured, distributed, or labeled by Innisfree Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
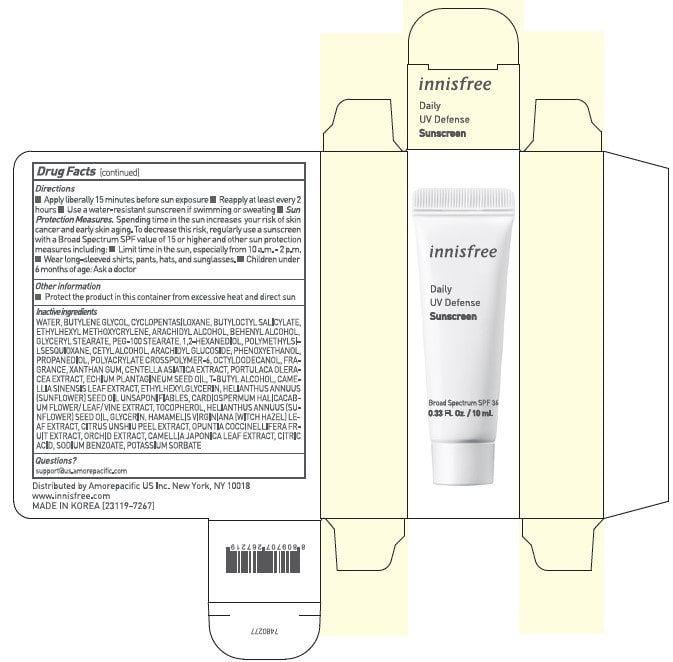
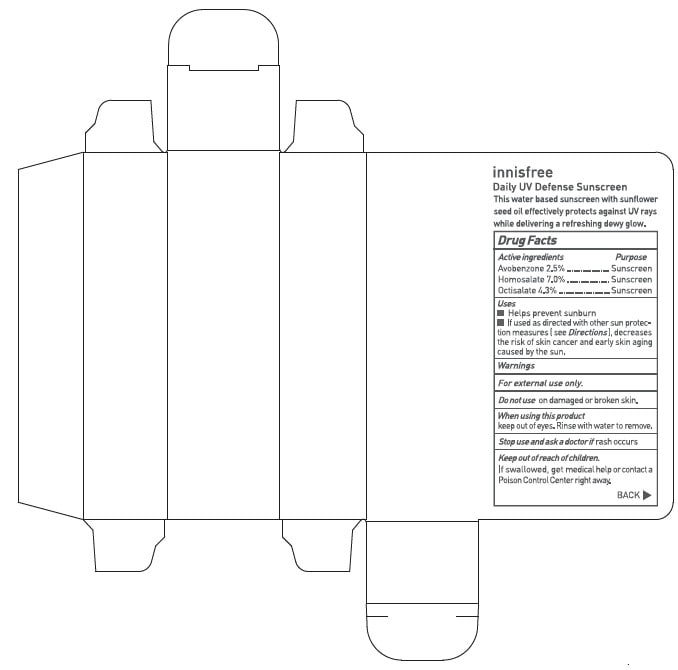
INNISFREE DAILY UV DEFENSE SUNSCREEN- avobenzone, homosalate, and octisalate lotion lotion
Innisfree Corporation
----------
innisfree Daily UV Defense Sunscreen
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging causes by the sun
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including :
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Children under 6 months of age : Ask a doctor.
Inactive ingredients
WATER / AQUA / EAU, BUTYLENE GLYCOL, CYCLOPENTASILOXANE, BUTYLOCTYL SALICYLATE, ETHYLHEXYL METHOXYCRYLENE, ARACHIDYL ALCOHOL, BEHENYL ALCOHOL, GLYCERYL STEARATE, PEG-100 STEARATE, 1,2-HEXANEDIOL, POLYMETHYLSILSESQUIOXANE, CETYL ALCOHOL, ARACHIDYL GLUCOSIDE, PHENOXYETHANOL, PROPANEDIOL, POLYACRYLATE CROSSPOLYMER-6, OCTYLDODECANOL, FRAGRANCE / PARFUM, XANTHAN GUM, CENTELLA ASIATICA EXTRACT, PORTULACA OLERACEA EXTRACT, ECHIUM PLANTAGINEUM SEED OIL, T-BUTYL ALCOHOL, CAMELLIA SINENSIS LEAF EXTRACT, ETHYLHEXYLGLYCERIN, HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL UNSAPONIFIABLES, CARDIOSPERMUM HALICACABUM FLOWER/LEAF/VINE EXTRACT, TOCOPHEROL, HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL, GLYCERIN, HAMAMELIS VIRGINIANA (WITCH HAZEL) LEAF EXTRACT, CITRUS UNSHIU PEEL EXTRACT, OPUNTIA COCCINELLIFERA FRUIT EXTRACT, ORCHID EXTRACT, CAMELLIA JAPONICA LEAF EXTRACT, CITRIC ACID, SODIUM BENZOATE, POTASSIUM SORBATE
Innisfree Daily UV Defense
innisfree
Daily
UV Defense
Sunscreen
Broad Spectrum SPF 36
1.69 Fl. Oz. / 50 mL

Innisfree Daily UV Defense - deluxe sample
innisfree
Daily
UV Defense
Sunscreen
Broad Spectrum SPF 36
0.33 Fl. Oz. / 10 mL
Innisfree Daily UV Defense - 100mL Jumbo
Innisfree
Daily
UV Defense
Sunscreen
Broad Spectrum SPF 36
3.38 Fl. Oz. /100 mL

| INNISFREE DAILY UV DEFENSE SUNSCREEN
avobenzone, homosalate, and octisalate lotion lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Innisfree Corporation (557822425) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

