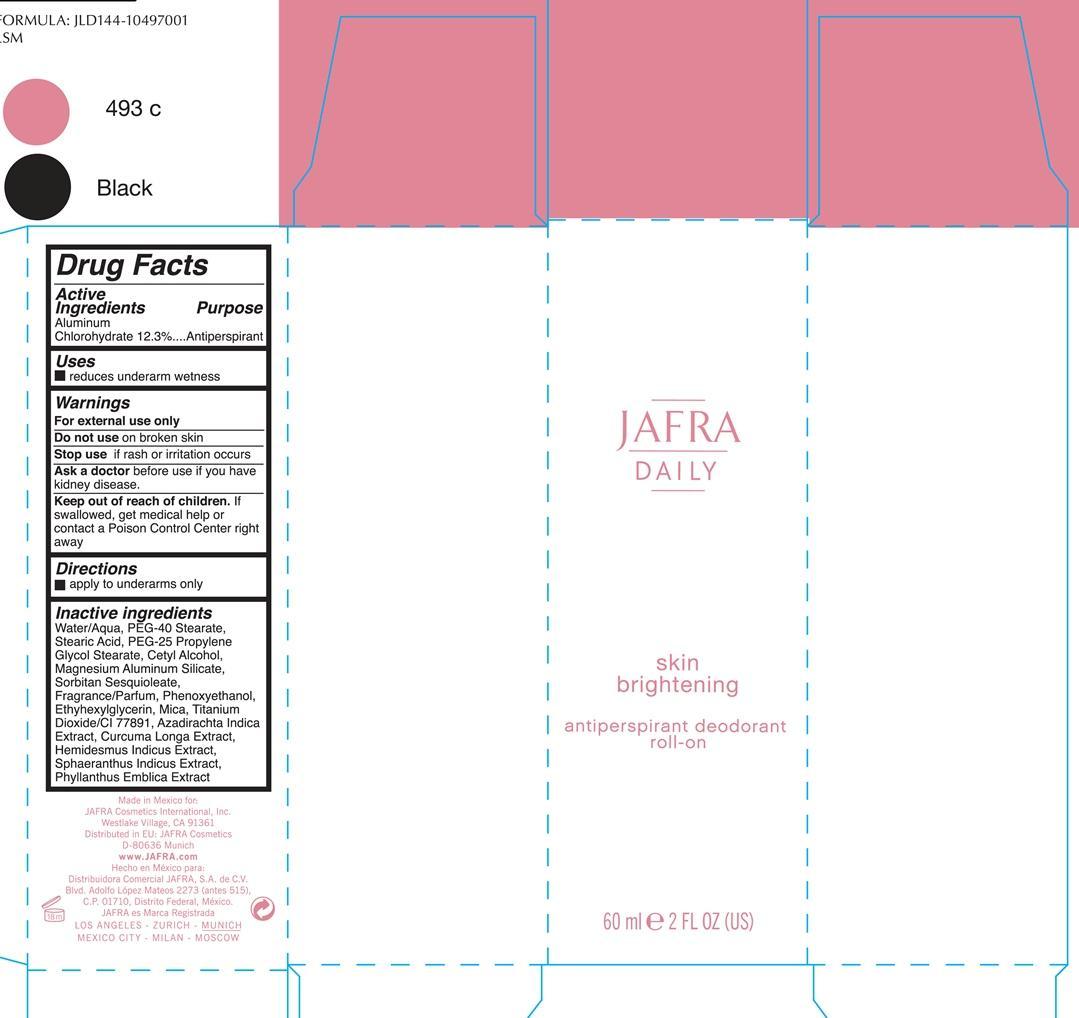
SKIN BRIGHTENING ANTIPERSPIRANT DEODORANT ROLL-ON JAFRA DAILY- aluminum chlorohydrate liquid
Skin brightening antiperspirant deodorant roll-on by
Drug Labeling and Warnings
Skin brightening antiperspirant deodorant roll-on by is a Otc medication manufactured, distributed, or labeled by Jafra cosmetics International, Jafra Manufacturing, S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENT
Active ingredients Purpose
Aluminum Chlorohydrate 12.3% Antiperspirant
Uses
reduces underarm wetness
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Stop use if rash or irritation occurs
Warnings
For external use only
Do not use on broken skin
Ask a doctor before before use if you have kidney disease
Directions
Apply to underarms only
Water/Aqua, PEG-40 Stearate, PEG-25 Propylene Glycol Stearate, Cetyl Alcohol, Magnesium Aluminum Silicate, Sorbitan Sesquioleate, Fragrance/parfum, Phenoxyethanol, Ehtylhexylhexyl Glycerin, Mica, Titanium Dioxide/CI 77891, Azadirachta Indica Extract, Curcuma Longa, Hemisdesmus Indicus, Sphaeranthus Indicus Extract, Phyllanthus Emblica Extract
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SKIN BRIGHTENING ANTIPERSPIRANT DEODORANT ROLL-ON JAFRA DAILY
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68828-199 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 12.3 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PEG-40 STEARATE (UNII: ECU18C66Q7) STEARIC ACID (UNII: 4ELV7Z65AP) PEG-25 PROPYLENE GLYCOL STEARATE (UNII: X21KPH4633) GLYCOL STEARATE (UNII: 0324G66D0E) CETYL ALCOHOL (UNII: 936JST6JCN) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) AZADIRACHTA INDICA BARK (UNII: G580B439YI) TURMERIC (UNII: 856YO1Z64F) HEMIDESMUS INDICUS ROOT (UNII: Y5CFT48S90) SPHAERANTHUS INDICUS FLOWERING TOP (UNII: 1O5Y93LB44) PHYLLANTHUS EMBLICA FRUIT (UNII: YLX4CW2576) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68828-199-02 1 in 1 CARTON 09/03/2014 1 NDC: 68828-199-01 60 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 09/03/2014 Labeler - Jafra cosmetics International (041676479) Registrant - Jafra cosmetics International (041676479) Establishment Name Address ID/FEI Business Operations Jafra Manufacturing, S.A. de C.V. 814732061 manufacture(68828-199)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.