TOPOTECAN injection, solution, concentrate
Topotecan by
Drug Labeling and Warnings
Topotecan by is a Prescription medication manufactured, distributed, or labeled by Teva Parenteral Medicines, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TOPOTECAN INJECTION safely and effectively. See full prescribing information for TOPOTECAN INJECTION.
TOPOTECAN injection, for intravenous use
Initial U.S. Approval: 1996WARNING: MYELOSUPPRESSION
See full prescribing information for complete boxed warning.
Topotecan can cause severe myelosuppression. Administer first cycle only to patients with baseline neutrophil counts greater than or equal to 1,500/mm3 and platelet counts greater than or equal to 100,000/mm3. Monitor blood cell counts (2.4, 5.1).
INDICATIONS AND USAGE
Topotecan Injection is a topoisomerase inhibitor indicated for treatment of:
- Patients with metastatic ovarian cancer after disease progression on or after initial or subsequent chemotherapy, as a single agent (1.1)
- Patients with small cell lung cancer (SCLC) platinum-sensitive disease who progressed at least 60 days after initiation of first-line chemotherapy, as a single agent (1.2)
- Patients with Stage IV-B, recurrent, or persistent cervical cancer which is not amenable to curative treatment, in combination with cisplatin (1.3)
DOSAGE AND ADMINISTRATION
- Ovarian Cancer and Small Cell Lung Cancer: 1.5 mg/m2 by intravenous infusion over 30 minutes daily for 5 consecutive days, starting on Day 1 of a 21 day cycle (2.1, 2.2)
- Cervical Cancer: 0.75 mg/m2 by intravenous infusion over 30 minutes on Days 1, 2, and 3, with cisplatin 50 mg/m2 on Day 1, of a 21 day cycle (2.3)
- Renal Impairment: Reduce dose if creatinine clearance (CLcr) 20 to 39 mL/min (2.6)
DOSAGE FORMS AND STRENGTHS
Injection: 4 mg/4 mL (1 mg/mL topotecan free base solution in a single-dose vial (3)
CONTRAINDICATIONS
- History of severe hypersensitivity reactions to topotecan (4)
WARNINGS AND PRECAUTIONS
- Interstitial Lung Disease (ILD): Fatal cases have occurred. Permanently discontinue if ILD confirmed. (5.2)
- Extravasation and Tissue Injury: Severe cases have occurred. If extravasation occurs, immediately stop administration and institute recommended management procedures. (5.3)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise patients of potential risk to the fetus and to use effectives contraception. (5.4, 8.1, 8.3)
ADVERSE REACTIONS
Ovarian Cancer
- The most common Grade 3 or 4 hematologic adverse reactions (incidence > 5%) were neutropenia, anemia, thrombocytopenia, and febrile neutropenia. (6.1)
- The most common (incidence > 5%) non-hematologic adverse reactions (all Grades) were nausea, vomiting, fatigue, diarrhea, and dyspnea. (6.1)
SCLC
- The most common Grade 3 or 4 hematologic adverse reactions were (incidence > 5%) neutropenia, anemia, thrombocytopenia, and febrile neutropenia. (6.1)
- The most common (incidence > 5%) non-hematologic adverse reactions (all Grades) were asthenia, dyspnea, nausea, pneumonia, abdominal pain, and fatigue. (6.1)
Cervical Cancer
- The most common Grade 3 or 4 hematologic adverse reactions were (incidence > 5%) neutropenia, anemia, and thrombocytopenia. (6.1)
- The most common (incidence > 25% and ≥ 2% higher than cisplatin alone) non-hematologic adverse reactions were pain, vomiting, and infection/febrile neutropenia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals USA, Inc. at 1-866-832-8537 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: MYELOSUPPRESSION
1 INDICATIONS AND USAGE
1.1 Ovarian Cancer
1.2 Small Cell Lung Cancer
1.3 Cervical Cancer
2 DOSAGE AND ADMINISTRATION
2.1 Important Safety Information
2.2 Recommended Dosage for Ovarian Cancer
2.3 Recommended Dosage for Small Cell Lung Cancer (SCLC)
2.4 Recommended Dosage for Cervical Cancer
2.5 Dosage Modifications for Adverse Reactions
2.6 Dosage Modification for Renal Impairment
2.7 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
5.2 Interstitial Lung Disease
5.3 Extravasation and Tissue Injury
5.4 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Ovarian Cancer
14.2 Small Cell Lung Cancer
14.3 Cervical Cancer
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: MYELOSUPPRESSION
Topotecan can cause severe myelosuppression. Administer first cycle only to patients with baseline neutrophil counts of greater than or equal to 1,500/mm3 and platelet counts greater than or equal to 100,000/mm3. Monitor blood cell counts [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
1.1 Ovarian Cancer
Topotecan Injection, as a single agent, is indicated for the treatment of patients with metastatic ovarian cancer after disease progression on or after initial or subsequent chemotherapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Safety Information
Calculate dosage using body surface area. Do not exceed a single dose of 4 mg intravenously.
2.2 Recommended Dosage for Ovarian Cancer
The recommended dosage of Topotecan Injection is 1.5 mg/m2 by intravenous infusion over 30 minutes daily for 5 consecutive days, starting on Day 1 of a 21 day cycle until disease progression or unacceptable toxicity.
2.3 Recommended Dosage for Small Cell Lung Cancer (SCLC)
The recommended dosage of Topotecan Injection is 1.5 mg/m2 by intravenous infusion over 30 minutes daily for 5 consecutive days, starting on Day 1 of a 21 day cycle.
2.4 Recommended Dosage for Cervical Cancer
The recommended dosage of Topotecan Injection is 0.75 mg/m2 by intravenous infusion over 30 minutes daily on Days 1, 2, and 3, in combination with cisplatin 50 mg/m2 on Day 1, of a 21 day cycle.
2.5 Dosage Modifications for Adverse Reactions
Hematologic
Withhold subsequent cycles of Topotecan Injection until neutrophils recover to greater than 1,000/mm3, platelets recover to greater than 100,000/mm3, and hemoglobin levels recover to greater than or equal to 9 g/dL (with transfusion if necessary).
For Topotecan Injection as a single agent, reduce the dose to 1.25 mg/m2/day for:
- neutrophil counts of less than 500/mm3 or administer granulocyte-colony stimulating factor (G-CSF) starting no sooner than 24 hours following the last dose
- platelet counts less than 25,000/mm3 during previous cycle
For Topotecan Injection in combination with cisplatin, reduce the dose to 0.6 mg/m2/day (and further to 0.45 mg/m2 if necessary) for:
- febrile neutropenia (defined as neutrophil counts less than 1,000/mm3 with temperature of greater than or equal to 38.0°C (100.4°F) or administer G-CSF starting no sooner than 24 hours following the last dose
- platelet counts less than 25,000/mm3 during previous cycle
2.6 Dosage Modification for Renal Impairment
For Topotecan Injection as a single agent, reduce the dose to 0.75 mg/m2/day for patients with creatinine clearance (CLcr) of 20 to 39 mL/min (calculated with the Cockcroft-Gault method using ideal body weight) [see Clinical Pharmacology (12.3)].
2.7 Preparation and Administration
- Topotecan Injection is a cytotoxic. Follow applicable special handling and disposable procedures.1
- Withdraw the required volume from the vial and discard any unused portion.
- Dilute Topotecan Injection in either 0.9% Sodium Chloride, USP or 5% Dextrose in Water, USP prior to administration.
- Store diluted Topotecan Injection solutions at approximately 20°C to 25°C (68°F to 77°F) for no more than 4 hours or under refrigerated [2°C to 8°C (36°F to 46°F)] conditions for no more than 12 hours.
- Visually inspect for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard if particulate matter or discoloration is observed.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Topotecan Injection is contraindicated in patients who have a history of severe hypersensitivity reactions to topotecan. Reactions have included anaphylactoid reactions [see Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
Topotecan can cause severe myelosuppression.
Single Agent
Grade 4 neutropenia occurred in 78% of 879 patients, with a median duration of 7 days and was most common during Cycle 1 (58% of patients). Grade 4 neutropenia associated with infection occurred in 13% and febrile neutropenia occurred in 5%. Sepsis occurred in 4% of patients and was fatal in 1%. Grade 4 thrombocytopenia occurred in 27%, with a median duration of 5 days. Grade 3 or 4 anemia occurred in 37% of patients.
Combination with Cisplatin
Grade 4 neutropenia occurred in 48% and Grade 4 thrombocytopenia occurred in 7% of 147 patients. Grade 3 or 4 anemia occurred in 40% of patients.
Topotecan can cause fatal typhlitis (neutropenic enterocolitis). Consider the possibility of typhlitis in patients presenting with fever, neutropenia, and abdominal pain.
Administer the first cycle of Topotecan Injection only to patients with a baseline neutrophil count of greater than or equal to 1,500/mm3 and a platelet count greater than or equal to 100,000/mm3. Monitor blood counts frequently during treatment. Withhold and reduce dose of Topotecan Injection based on neutrophil counts, platelet counts and hemoglobin levels [see Dosage and Administration (2.5)].
5.2 Interstitial Lung Disease
Interstitial lung disease (ILD), including fatalities, can occur with topotecan. Underlying risk factors include history of ILD, pulmonary fibrosis, lung cancer, thoracic radiation, and use of pneumotoxic drugs or colony stimulating factors. Monitor for pulmonary symptoms indicative of ILD. Permanently discontinue Topotecan Injection if ILD is confirmed.
5.3 Extravasation and Tissue Injury
Extravasation, including severe cases, can occur with topotecan. If signs or symptoms of extravasation occur, immediately stop administration of Topotecan Injection and institute recommended management procedures [see Adverse Reactions (6.1)].
5.4 Embryo-Fetal Toxicity
Based on animal data, Topotecan Injection can cause fetal harm when administered to a pregnant woman. Topotecan caused embryolethality, fetotoxicity, and teratogenicity in rats and rabbits when administered during organogenesis. Advise women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 6 months after the last dose of Topotecan Injection. Advise males with a female partner of reproductive potential to use effective contraception during treatment with Topotecan Injection and for 3 months after the last dose [see Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Myelosuppression [see Warnings and Precautions (5.1)]
- Interstitial Lung Disease [see Warnings and Precautions (5.2)]
- Extravasation and Tissue Injury [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data in Warnings and Precautions reflect exposure to topotecan from eight trials in which 879 patients with ovarian cancer or small cell lung cancer (SCLC) received topotecan 1.5 mg/m2 by intravenous infusion daily for 5 consecutive days, starting on Day 1 of a 21 day cycle and from one trial (Study GOG 0179) in which 147 patients with cervical cancer received topotecan 0.75 mg/m2 by intravenous infusion daily on Days 1, 2, and 3, with cisplatin 50 mg/m2 by intravenous infusion on Day 1, of a 21 day cycle.
Ovarian Cancer
The safety of topotecan was evaluated in a randomized trial conducted in 226 patients with metastatic ovarian cancer (Study 039) [see Clinical Studies (14.1)]. Table 1 shows the incidence of Grade 3 and 4 hematologic and non-hematologic adverse reactions that occurred in patients receiving topotecan.
Table 1. Adverse Reactions Occurring in ≥ 5% of Patients with Ovarian Cancer in Study 039 Adverse Reaction
Topotecan
(n = 112)
Paclitaxel (n = 114)
Grade 3 to 4 (%)
Grade 3 to 4 (%)
Hematologic
Grade 4 neutropenia (< 500/mm3)
80
21
Grade 3 or 4 anemia (Hgb < 8 g/dL)
Grade 4 thrombocytopenia (< 25,000/mm3)
41
27
6
3
Febrile neutropenia
23
4
Non-Hematologic
Infections
Sepsisa
5
2Respiratory, thoracic, and mediastinal
Dyspnea
6
5Gastrointestinal
10
3Vomiting
Nausea
10
2
Diarrhea
6
1
Abdominal pain
5
4
Intestinal obstruction
5
4
Constipation
5
0
General and administrative site conditions
7
6Fatigue
Painb
5
7
Asthenia
5
3
a Death related to sepsis occurred in 2% of patients receiving topotecan and 0% of patients receiving paclitaxel.
b Pain includes body pain, skeletal pain, and back pain.
Small Cell Lung Cancer (SCLC)
The safety of topotecan was evaluated in randomized, comparative trial in patients with recurrent or progressive SCLC (Study 090) [see Clinical Studies (14.2)]. Table 2 shows the Grade 3 or 4 hematologic and non-hematologic adverse reactions in patients with SCLC.
Table 2. Adverse Reactions Occurring in ≥ 5% of Patients with Small Cell Lung Cancer in Study 090 Adverse Reactions
Topotecan
(n = 107)
CAVc (n = 104)
Grades 3 to 4 (%)
Grades 3 to 4 (%)
Hematologic
Grade 4 neutropenia (< 500/mm3)
70
72
Grade 3 or 4 anemia (Hgb < 8 g/dL)
Grade 4 thrombocytopenia (< 25,000/mm3)
42
29
20
5
Febrile neutropenia
28
26
Non-Hematologic
Infections
Sepsisa
5
5Respiratory, thoracic, and mediastinal
9
14Dyspnea
Pneumonia
8
6
Gastrointestinal
8
6Nausea
Abdominal pain
6
4
General and administrative site conditions
9
7Asthenia
Fatigue
6
10
Painb
5
7
a Death related to sepsis occurred in 3% of patients receiving topotecan and 1% of patients receiving CAV.
b Pain includes body pain, skeletal pain, and back pain.
c CAV = cyclophosphamide, doxorubicin and vincristine.
Hepatobiliary Disorders in Ovarian and Small Cell Lung Cancer
Based on the combined experience of 453 patients with metastatic ovarian cancer and 426 patients with SCLC treated with topotecan, Grade 3 or 4 increases aspartate transaminase (AST) or alanine transaminase (ALT) occurred in 4% and Grade 3 or 4 elevated bilirubin occurred in less than 2%.
Cervical Cancer
The safety of topotecan was evaluated in a comparative trial of topotecan with cisplatin versus cisplatin as a single agent in patients with cervical cancer (Study GOG 0179). Table 3 shows the hematologic and non-hematologic adverse reactions in patients with cervical cancer.
Table 3. Adverse Reactions Occurring in ≥ 5% of Patients with Cervical Cancer (Between-Arm Difference ≥ 2%)a in Study GOG 0179 Adverse Reaction
Topotecan
with Cisplatin
(n = 140)
%
Cisplatin (n = 144)
%
Hematologic
Neutropenia
26
1Grade 3 (< 1,000 to 500/mm3)
Grade 4 (< 500/mm3)
48
1
Anemia
34
19Grade 3 (Hgb < 8 to 6.5 g/dL)
Grade 4 (Hgb < 6.5 g/dL)
6
3
Thrombocytopenia
26
3Grade 3 (< 50,000 to 10,000/mm3)
Grade 4 (< 10,000/mm3)
7
0
Non-Hematologicb,c
General and administrative site conditions
69
62Constitutionald
Paine
59
50
Gastrointestinal
40
37Vomiting
Stomatitis-pharyngitis
6
0
Other
63
56
Dermatologyf
48
20
Infection
Febrile neutropeniaf
28
18
Cardiovascularf
25
15
a Includes patients who were eligible and treated.
b Severity based on using National Cancer Institute (NCI) Common Toxicity Criteria (CTC), Version 2.0.
c Grades 1 through 4 only. There were 3 patients who experienced deaths with investigator-designated attribution. The first patient experienced a Grade 5 hemorrhage in which the drug-related thrombocytopenia aggravated the event. A second patient experienced bowel obstruction, cardiac arrest, pleural effusion, and respiratory failure which were not treatment-related but probably aggravated by treatment. A third patient experienced a pulmonary embolism and adult respiratory distress syndrome; the latter was indirectly treatment-related.
d Constitutional includes fatigue (lethargy, malaise, asthenia), fever (in the absence of neutropenia), rigors, chills, sweating, and weight gain or loss.
e Pain includes abdominal pain or cramping, arthralgia, bone pain, chest pain (non-cardiac and non-pleuritic), dysmenorrhea, dyspareunia, earache, headache, hepatic pain, myalgia, neuropathic pain, pain due to radiation, pelvic pain, pleuritic pain, rectal or perirectal pain, and tumor pain.
f High-level terms were included if the between-arm difference was ≥ 10%.
6.2 Postmarketing Experience
The following reactions have been identified during postapproval use of topotecan. Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System: severe bleeding (in association with thrombocytopenia)
Hypersensitivity: allergic manifestations, anaphylactoid reactions, angioedema
Gastrointestinal: abdominal pain potentially associated with neutropenic enterocolitis, gastrointestinal perforation
Pulmonary: interstitial lung disease
Skin and Subcutaneous Tissue: severe dermatitis, severe pruritus
General and Administration Site Conditions: extravasation, mucosal inflammation
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal data and its mechanism of action, Topotecan Injection can cause fetal harm when administered to a pregnant woman. There are no available clinical data on the use of topotecan in pregnancy. Topotecan caused embryolethality, fetotoxicity, and teratogenicity in rats and rabbits when administered during organogenesis at doses similar to the clinical dose (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the background risk of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Data
Animal Data
In rabbits, an intravenous dose of 0.10 mg/kg/day [about equal to the 1.5 mg/m2 clinical dose based on body surface area (BSA)] given on Days 6 through 20 of gestation caused maternal toxicity, embryolethality and reduced fetal body weight. In the rat, an intravenous dose of 0.23 mg/kg/day (about equal to the 1.5 mg/m2 clinical dose based on BSA) given for 14 days before mating through gestation Day 6 caused fetal resorption, microphthalmia, pre-implant loss, and mild maternal toxicity. Administration of an intravenous dose of 0.10 mg/kg/day (about half the 1.5 mg/m2 clinical dose based on BSA) given to rats on Days 6 through 17 of gestation caused an increase in post-implantation mortality. This dose also caused an increase in total fetal malformations. The most frequent malformations were of the eye (microphthalmia, anophthalmia, rosette formation of the retina, coloboma of the retina, ectopic orbit), brain (dilated lateral and third ventricles), skull, and vertebrae.
8.2 Lactation
Risk Summary
There are no data on the presence of topotecan or its metabolites in human milk or their effects on the breastfed infant or on milk production. Lactating rats excrete high concentrations of topotecan in milk (see Data). Because of the potential for serious adverse reactions in breastfed infants, advise women not to breastfeed during treatment with Topotecan Injection and for 1 week after the last dose.
Data
Animal Data
Following intravenous administration of topotecan to lactating rats at a dose of 4.72 mg/m2 (about twice the 1.5 mg/m2 clinical dose based on BSA), topotecan was excreted into milk at concentrations up to 48 fold higher than those in plasma.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status of females of reproductive potential prior to initiating Topotecan Injection [see Use in Specific Populations (8.1)].
Contraception
Topotecan Injection can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Females
Advise females of reproductive potential to use effective contraception during treatment with Topotecan Injection and for 6 months after the last dose.
Males
Topotecan may damage spermatozoa, resulting in possible genetic and fetal abnormalities. Advise males with a female partner of reproductive potential to use effective contraception during treatment with Topotecan Injection and for 3 months after the last dose [see Nonclinical Toxicology (13.1)].
Infertility
Females
Topotecan can have both acute and long-term effects on fertility [see Nonclinical Toxicology (13.1)].
Males
Effects on spermatogenesis occurred in animals administered topotecan [see Nonclinical Toxicology (13.1)].
8.5 Geriatric Use
Of the 879 patients with metastatic ovarian cancer or small cell lung cancer in clinical trials of topotecan, 32% were aged 65 years and older, while 3.8% were aged 75 years and older. Of the 140 patients with Stage IV-B, relapsed or refractory cervical cancer in clinical trials of topotecan who received topotecan with cisplatin in the randomized clinical trial, 6% were aged 65 years and older, while 3% were aged 75 years and older. No overall differences in effectiveness or safety were observed between these patients and younger patients and other reported clinical experience has not identified differences in responses between the elderly and younger patients.
8.6 Renal Impairment
Reduce the dose of Topotecan Injection in patients with a CLcr of 20 to 39 mL/min [see Dosage and Administration (2.6), Clinical Pharmacology (12.3)]. No dosage adjustment is recommended for patients with CLcr greater than or equal to 40 mL/min. Insufficient data are available in patients with CLcr less than 20 mL/min to provide a dosage recommendation for Topotecan Injection.
-
10 OVERDOSAGE
Overdoses (up to 10-fold of the recommended dose) have occurred in patients receiving intravenous topotecan. The primary complication of overdosage is myelosuppression. Elevated hepatic enzymes, mucositis, gastrointestinal toxicity, and skin toxicity have occurred with overdosages. If an overdose is suspected, monitor the patient closely for myelosuppression and institute supportive-care measures as appropriate.
-
11 DESCRIPTION
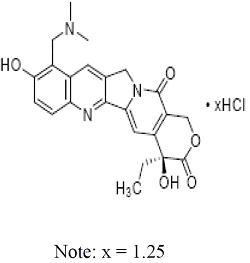
Topotecan is a semi-synthetic derivative of camptothecin and a topoisomerase inhibitor. The chemical name for topotecan hydrochloride is (S)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3’,4’:6,7] indolizino [1,2-b]quinoline-3,14-(4H,12H)-dione 1.25 hydrochloride. It has the molecular formula of C23H23N3O5xHCl (x = 1.25) and a molecular weight of 467.02. It is soluble in water and melts with decomposition at 213°C to 218°C.
Topotecan hydrochloride has the following structural formula:
Topotecan Injection for intravenous use is supplied as a sterile, non-pyrogenic, clear, light yellow to greenish solution in a single-dose vial at a topotecan free base concentration of 4 mg/4 mL (1 mg/mL).
Each mL contains 1 mg topotecan free base (equivalent to 1.11 mg topotecan hydrochloride), 12 mg of mannitol, USP, and 5 mg of tartaric acid, NF. It may also contain hydrochloric acid and sodium hydroxide to adjust the pH. The solution pH ranges from 2.0 to 2.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Topoisomerase I relieves torsional strain in DNA by inducing reversible single-strand breaks. Topotecan binds to the topoisomerase I-DNA complex and prevents re-ligation of these single-strand breaks. The cytotoxicity of topotecan is thought to be due to double-strand DNA damage produced during DNA synthesis, when replication enzymes interact with the ternary complex formed by topotecan, topoisomerase I, and DNA. Mammalian cells cannot efficiently repair these double-strand breaks.
12.3 Pharmacokinetics
Following administration of topotecan at doses of 0.5 to 1.5 mg/m2 (0.1 to 0.3 times the recommended single agent dose) administered as a 30-minute infusion, the area under the curve (AUC) increases proportionally with dose.
Distribution
Protein binding of topotecan is approximately 35%.
Elimination
The terminal half-life of topotecan is 2 to 3 hours following intravenous administration.
Metabolism
Topotecan undergoes a reversible pH-dependent hydrolysis of its pharmacologically active lactone moiety. At pH less than or equal to 4, the lactone is exclusively present, whereas the ring-opened hydroxy-acid form predominates at physiologic pH. Topotecan is metabolized to an N-demethylated metabolite in vitro. The mean metabolite: parent AUC ratio is 3% for total topotecan and topotecan lactone following intravenous administration.
Excretion
The overall recovery of total topotecan and its N-desmethyl metabolite in urine and feces over 9 days averaged 73% ± 2% following an intravenous dose. Mean values (±SD) of 51% (± 3%) as total topotecan and 3% (± 1%) as N-desmethyl topotecan were excreted in the urine. Fecal elimination of total topotecan accounted for 18% (± 4%) while fecal elimination of N-desmethyl topotecan was 1.7% (± 0.6%). An O-glucuronidation metabolite of topotecan and N-desmethyl topotecan has been identified in the urine.
Specific Populations
No clinically significant differences in the pharmacokinetics of topotecan were observed based on age, sex, or hepatic impairment following intravenous administration.
Patients with Renal Impairment
Compared to patients with CLcr (calculated by the Cockcroft-Gault method using ideal body weight) greater than 60 mL/min, plasma clearance of topotecan lactone decreased by 33% in patients with CLcr 40 to 60 mL/min and decreased 65% in patients with CLcr 20 to 39 mL/min. The effect on topotecan pharmacokinetics in patients with CLcr less than 20 mL/min is unknown [see Dosage and Administration (2.6)].
Drug Interaction Studies
Clinical Studies
No clinically significant changes in topotecan pharmacokinetics were observed when coadministered cisplatin with topotecan.
No clinically significant changes in the pharmacokinetics of free platinum were observed in patients coadministered cisplatin with topotecan.
In Vitro Studies
Topotecan does not inhibit CYP1A2, CYP2A6, CYP2C8/9, CYP2C19, CYP2D6, CYP2E, CYP3A, or CYP4A or dihydropyrimidine dehydrogenase.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity testing of topotecan has not been performed. Topotecan is known to be genotoxic to mammalian cells and is a probable carcinogen. Topotecan was mutagenic to L5178Y mouse lymphoma cells and clastogenic to cultured human lymphocytes with and without metabolic activation. It was also clastogenic to mouse bone marrow. Topotecan did not cause mutations in bacterial cells.
Topotecan given to female rats prior to mating at an intravenous dose of 1.4 mg/m2 [about equal to the clinical dose based on body surface area (BSA)] caused superovulation possibly related to inhibition of follicular atresia. This dose given to pregnant female rats also caused increased pre-implantation loss. Studies in dogs given at an intravenous dose of 0.4 mg/m2 (about 0.25 times the clinical dose based on BSA) of topotecan daily for a month suggest that treatment may cause an increase in the incidence of multinucleated spermatogonial giant cells in the testes.
-
14 CLINICAL STUDIES
14.1 Ovarian Cancer
The efficacy of topotecan was evaluated in two clinical trials of 223 patients with metastatic ovarian cancer. All patients had disease that had recurred on, or was unresponsive to, a platinum-containing regimen. Patients in these trials received an initial dose of 1.5 mg/m2 as an intravenous infusion for 5 consecutive days, starting on Day 1 of a 21 day cycle.
One trial (Study 039) was a randomized trial of 112 patients who received topotecan and of 114 patients who received paclitaxel (175 mg/m2 intravenously over 3 hours on Day 1 of a 21 day cycle). All patients had recurrent ovarian cancer after a platinum-containing regimen or had not responded to at least 1 prior platinum-containing regimen. Patients who did not respond to the trial therapy, or who progressed, could be given the alternative treatment. The efficacy outcome measures were overall response rate, response duration, time to progression, and overall survival (OS).
The results of the trial did not show statistically significant improvements in response rates, response duration, time to progression, and OS as shown in Table 4.
Table 4. Efficacy Results in Ovarian Cancer in Study 039 Parameter
Topotecan
(n = 112)
Paclitaxel (n = 114)
Overall response rate (95% CI)
21% (13%, 28%)
14% (8%, 20%)
Complete response rate
5%
3%
Partial response rate
16%
11%
Response durationa (months)
Median (95% CI)
6 (5.1, 7.6)
5 (3.7, 7.8)
Time to progression (months)
Median (95% CI)
Hazard ratio (95% CI)
4.4 (2.8, 5.4) 3.4 (2.7, 4.2)0.76 (0.57, 1.02)
Overall survival (months)
14.5 (10.7, 16.5) 12.2 (9.7, 15.8)Median (95% CI)
Hazard ratio (95% CI)
0.97 (0.71, 1.34)
Abbreviation: CI, confidence interval.
a The calculation for response duration was based on the interval between first response and time to progression.
The median time to response was 7.6 weeks (3.1 weeks to 5 months) with topotecan compared with 6 weeks (2.4 weeks to 4.1 months) with paclitaxel. In the cross-over phase, 13% of 61 patients who received topotecan after paclitaxel had a partial response and 10% of 49 patients who received paclitaxel after topotecan had a response (2 complete responses).
Topotecan was active in ovarian cancer patients who had developed resistance to platinum-containing therapy, defined as tumor progression while on, or tumor relapse within 6 months after completion of, a platinum-containing regimen. One complete and 6 partial responses were seen in 60 patients, for a response rate of 12%. In the same trial, there were no complete responders and 4 partial responders on the paclitaxel arm, for a response rate of 7%.
Topotecan was also studied in an open-label, non-comparative trial in 111 patients with recurrent ovarian cancer after treatment with a platinum-containing regimen, or who had not responded to 1 prior platinum-containing regimen. The response rate was 14% (95% CI: 7%, 20%). The median duration of response was 5 months (4.6 weeks to 9.6 months). The time to progression was 2.6 months (5 days to 1.4 years). The median survival was 1.3 years (1.4 weeks, to 2.2 years).
14.2 Small Cell Lung Cancer
The efficacy of topotecan was evaluated in 426 patients with recurrent or progressive small cell lung cancer (SCLC) in a randomized, comparative trial and in 3 single-arm trials.
Randomized Comparative Trial
In a randomized, comparative trial, 211 patients were randomized 1:1 to receive topotecan (1.5 mg/m2 once daily intravenously for 5 days starting on Day 1 of a 21 day cycle) or CAV (cyclophosphamide 1,000 mg/m2, doxorubicin 45 mg/m2, vincristine 2 mg administered sequentially on Day 1 of a 21 day cycle). All patients were considered sensitive to first-line chemotherapy (responders who then subsequently progressed greater than or equal to 60 days after completion of first-line therapy). A total of 77% of patients treated with topotecan and 79% of patients treated with CAV received platinum/etoposide with or without other agents as first-line chemotherapy. The efficacy outcome measures were overall response rate, response duration, time to progression or OS.
The results of the trial did not show statistically significant improvements in response rate, response duration, time to progression, or OS as shown in Table 5.
Table 5. Efficacy Results in Patients with Small Cell Lung Cancer Sensitive to First-Line Chemotherapy in Study 090 Parameter
Topotecan
(n = 107)
CAVb
(n = 104)
Overall response rate (95% CI)
24% (16%, 32%)
18% (11%, 26%)
Complete response rate
0%
1%
Partial response rate
24%
17%
Response durationa (months)
Median (95% CI)
3.3 (3, 4.1)
3.5 (3, 5.3)
Time to progression (months)
3.1 (2.6, 4.1) 2.8 (2.5, 3.2)
Median (95% CI)
Hazard ratio (95% CI)
0.92 (0.69, 1.22)
Overall survival (months)
5.8 (4.7, 6.8) 5.7 (5, 7)
Median (95% CI)
Hazard ratio (95% CI)
1.04 (0.78, 1.39)
Abbreviations: CI, confidence interval.
a The calculation for duration of response was based on the interval between first response and time to progression.
b CAV = cyclophosphamide, doxorubicin and vincristine.
The median time to response was similar in both arms: topotecan, 6 weeks (2.4 weeks to 3.6 months) versus CAV, 6 weeks (5.1 weeks to 4.2 months).
Changes on a disease-related symptom scale are presented in Table 6. It should be noted that not all patients had all symptoms, nor did all patients respond to all questions. Each symptom was rated on a 4-category scale with an improvement defined as a change in 1 category from baseline sustained over 2 courses. Limitations in interpretation of the rating scale and responses preclude formal statistical analysis.
Table 6. Symptom Improvementa in Patients with Small Cell Lung Cancer in Study 090 Symptom
Topotecan
(n = 107)
CAV
(n = 104)
nb
(%)
nb
(%)
Shortness of breath
68
28
61
7
Interference with daily activity
67
27
63
11
Fatigue
70
23
65
9
Hoarseness
40
33
38
13
Cough
69
25
61
15
Insomnia
57
33
53
19
Anorexia
56
32
57
16
Chest pain
44
25
41
17
Hemoptysis
15
27
12
33
a Defined as improvement sustained over at least 2 courses compared with baseline.
b Number of patients with baseline and at least 1 post-baseline assessment.
Single-Arm Trials
Topotecan was also studied in three open-label, non-comparative trials (Studies 014, 092 and 053) in a total of 319 patients with recurrent or progressive SCLC after treatment with first-line chemotherapy. In all three trials, patients were stratified as either sensitive (responders who then subsequently progressed greater than or equal to 90 days after completion of first-line therapy) or refractory (no response to first-line chemotherapy or who responded to first-line therapy and then progressed within 90 days of completing first-line therapy). Response rates ranged from 11% to 31% for sensitive patients and 2% to 7% for refractory patients. Median time to progression and median survival were similar in all three trials and the comparative trial.
14.3 Cervical Cancer
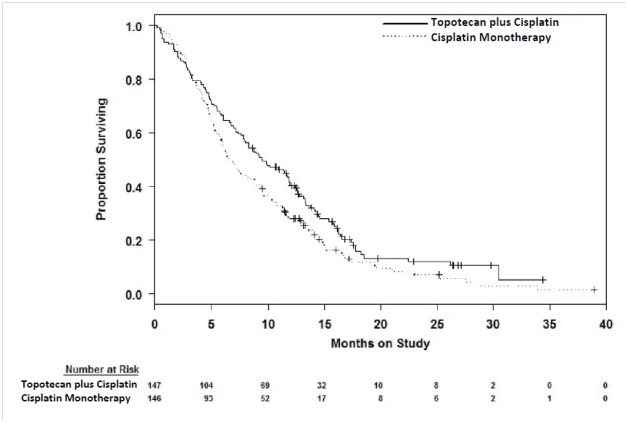
The efficacy of topotecan was evaluated in a multi-center, randomized (1:1), open-label study (Study GOG 0179) conducted in 147 patients with histologically confirmed Stage IV-B, recurrent, or persistent cervical cancer considered not amenable to curative treatment with surgery and/or radiation. Patients were randomized to topotecan (0.75 mg/m2 once daily intravenously for 3 consecutive days starting on Day 1 of a 21-day cycle) with cisplatin (50 mg/m2 intravenously on Day 1) or cisplatin as a single agent. Fifty-six percent of patients treated with topotecan with cisplatin and 56% of patients treated with cisplatin had received prior cisplatin with or without other agents as first-line chemotherapy. The efficacy outcome measure was OS.
Median OS of eligible patients receiving topotecan with cisplatin was 9.4 months (95% CI: 7.9, 11.9) compared with 6.5 months (95% CI: 5.8, 8.8) among patients randomized to cisplatin alone with a log rank P-value of 0.033 (significance level was 0.044 after adjusting for the interim analysis). The unadjusted hazard ratio for OS was 0.76 (95% CI: 0.59, 0.98).
Figure 1. Kaplan-Meier Curves for Overall Survival in Cervical Cancer in Study GOG 0179
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Topotecan Injection is supplied in 4 mg/4 mL (1 mg/mL topotecan free base) single-dose vials. Each vial contains 4 mL of the sterile, clear, light yellow to greenish solution.
NDC: 0703-4714-01 (Package of 1 Single-Dose Vial NDC: 0703-4714-71)
Store refrigerated between 2°C and 8°C (36°F and 46°F) in the original carton to protect from light. Discard unused portion.
Topotecan Injection is a cytotoxic drug. Follow applicable handling and disposal procedures.1
-
17 PATIENT COUNSELING INFORMATION
Myelosuppression
Advise patients of the risks of myelosuppression and instruct them to notify their healthcare provider promptly for fever, other signs of infection, or bleeding [see Warnings and Precautions (5.1)].
Interstitial Lung Disease (ILD)
Inform patients of the risks of severe ILD. Advise patients to contact their healthcare provider immediately to report new or worsening respiratory symptoms [see Warnings and Precautions (5.2)].
Embryo-Fetal Toxicity
Advise females of reproductive potential and males with female partners of reproductive potential of the potential risk to a fetus. Advise women to contact their healthcare provider if they become pregnant, or if pregnancy is suspected during treatment with Topotecan Injection [see Warnings and Precautions (5.4), Use in Specific Populations (8.1, 8.3)].
Advise females of reproductive potential to use effective contraception during treatment and for 6 months after the last dose of Topotecan Injection [see Use in Specific Populations (8.1, 8.3)].
Advise males with a female partner of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of Topotecan Injection [see Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)].
Lactation
Advise women to discontinue breastfeeding during treatment and for 1 week after the last dose of Topotecan Injection [see Use in Specific Populations (8.2)].
Infertility
Advise male and female patients of the potential risk for impaired fertility [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Asthenia and Fatigue
Advise patients of the risk of asthenia or fatigue. These symptoms may impair the ability to safely drive or operate machinery.
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454 -
Package/Label Display Panel
NDC: 0703-4714-01
Rx only
Topotecan
Injection
4 mg/4 mL
(1 mg/mL)
Must dilute before intravenous
infusion.
Store refrigerated between
2°C and 8°C (36°F and 46°F)
in the original carton to
protect from light.
Cytotoxic Agent
Single-Dose Vial. Discard unused
portion.
Sterile
TEVA
-
INGREDIENTS AND APPEARANCE
TOPOTECAN
topotecan injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0703-4714 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOPOTECAN HYDROCHLORIDE (UNII: 956S425ZCY) (TOPOTECAN - UNII:7M7YKX2N15) TOPOTECAN 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) TARTARIC ACID (UNII: W4888I119H) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color YELLOW (clear, light yellow to greenish) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0703-4714-01 1 in 1 CARTON 05/21/2013 1 NDC: 0703-4714-71 4 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022453 05/21/2013 Labeler - Teva Parenteral Medicines, Inc. (794362533)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.