HYDROCORTISONE by Encube Ethicals, Inc. / Encube Ethicals Private Limited HYDROCORTISONE cream
HYDROCORTISONE by
Drug Labeling and Warnings
HYDROCORTISONE by is a Otc medication manufactured, distributed, or labeled by Encube Ethicals, Inc., Encube Ethicals Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
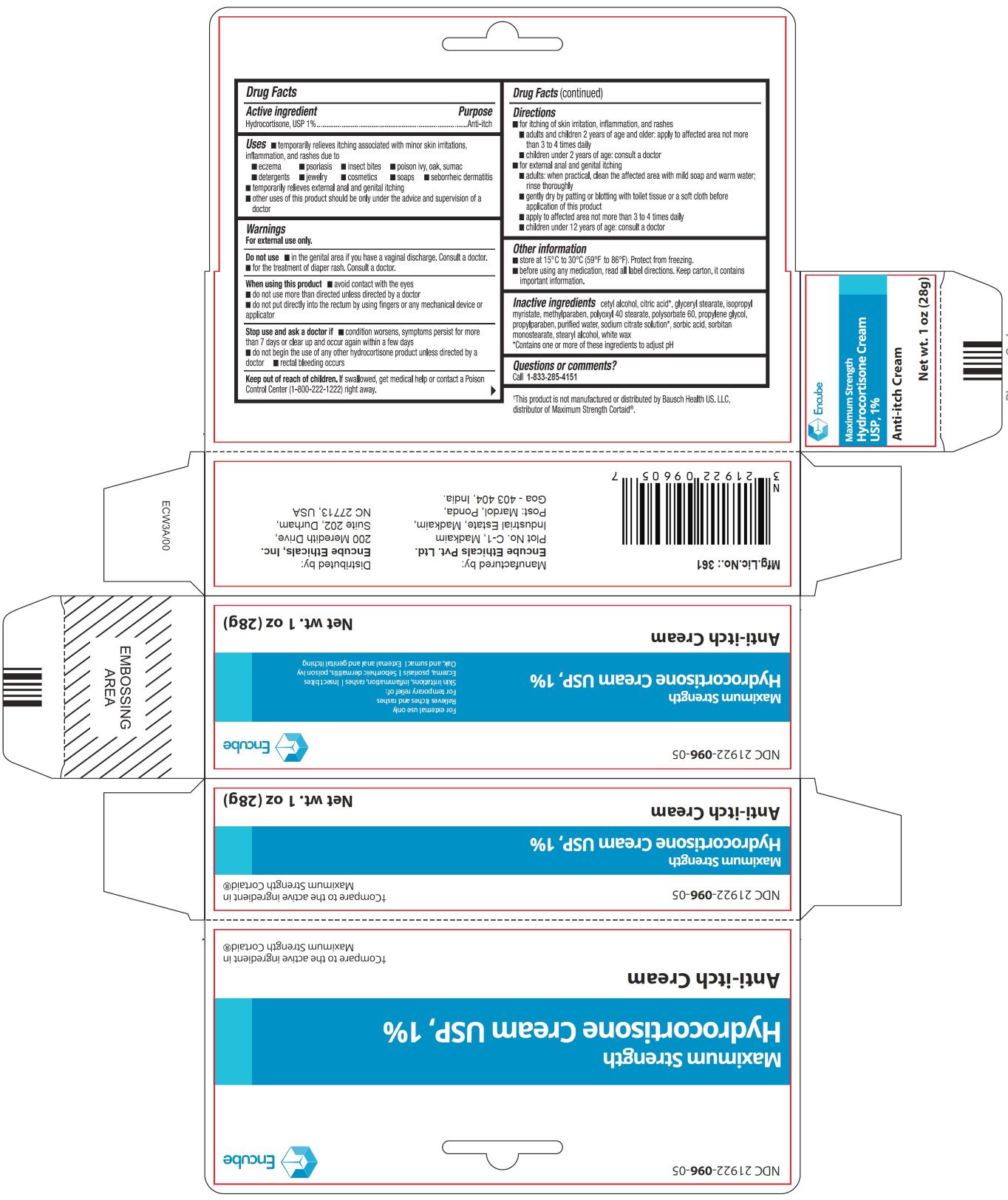
- ACTIVE INGREDIENT(S)
- PURPOSE
-
USE(S)
■temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to
■eczema ■ psoriasis ■ insect bites ■ poison ivy, oak, sumac
■detergents ■ jewelry ■ cosmetics ■ soaps ■ seborrheic dermatitis
■temporarily relieves external anal and genital itching
■other uses of this product should be only under the advice and supervision of a doctor
- WARNINGS
- DO NOT USE
- WHEN USING THIS PRODUCT
- STOP USE AND ASK DOCTOR IF
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
■ for itching of skin irritation, inflammation, and rashes
■ adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
■ children under 2 years of age: consult a doctor
■ for external anal and genital itching
■ adults: when practical, clean the affected area with mild soap and warm water; rinse thoroughly
■ gently dry by paling or blotting with toilet Issue or a soft cloth before application of this product
■ apply to affected area not more than 3 to 4 times daily
■ children under 12 years of age: consult a doctor
- OTHER INFORMATION
-
INACTIVE INGREDIENTS
cetyl alcohol, citric acid*, glyceryl stearate, isopropyl myristate, methylparaben, polyoxyl 40 stearate, polysorbate 60, propylene glycol, propylparaben, purified water, sodium citrate solution* sorbic acid, sorbitan monostearate, stearyl alcohol, white wax
*Contains one or more of these ingredients to adjust pH
- QUESTIONS OR COMMENTS?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 21922-096 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) METHYLPARABEN (UNII: A2I8C7HI9T) POLYOXYL 40 STEARATE (UNII: 13A4J4NH9I) POLYSORBATE 60 (UNII: CAL22UVI4M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) SORBIC ACID (UNII: X045WJ989B) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) WHITE WAX (UNII: 7G1J5DA97F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 21922-096-04 1 in 1 CARTON 02/25/2025 1 14 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 21922-096-05 1 in 1 CARTON 02/25/2025 2 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/25/2025 Labeler - Encube Ethicals, Inc. (116982244) Establishment Name Address ID/FEI Business Operations Encube Ethicals Private Limited 725076298 ANALYSIS(21922-096) , LABEL(21922-096) , MANUFACTURE(21922-096) , PACK(21922-096)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.