ETHYOL- amifostine injection, powder, lyophilized, for solution
Ethyol by
Drug Labeling and Warnings
Ethyol by is a Prescription medication manufactured, distributed, or labeled by Clinigen Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ETHYOL safely and effectively. See full prescribing information for ETHYOL.
ETHYOL® (amifostine) for injection, for intravenous use
Initial U.S. Approval: 1995INDICATIONS AND USAGE
ETHYOL is a cytoprotective agent indicated for:
- reduction of cumulative renal toxicity associated with repeated administration of cisplatin in patients with advanced ovarian cancer. (1.1)
- reduction of the incidence of moderate to severe xerostomia in patients undergoing post-operative radiation treatment for head and neck cancer, where the radiation port includes a substantial portion of the parotid glands. (1.2)
Limitation of Use
Avoid the use of ETHYOL in settings where chemotherapy can produce a significant survival benefit or cure, or in patients receiving definitive radiotherapy. (1, 5.1, 5.2)
DOSAGE AND ADMINISTRATION
- For reduction of cumulative renal toxicity with chemotherapy, the recommended starting dose is 910 mg/m2 administered once daily as a 15-minute intravenous infusion, starting 30 minutes prior to chemotherapy. (2.1)
- For reduction of moderate to severe xerostomia from radiation of the head and neck, the recommended dose is 200 mg/m2 administered once daily as a 3-minute intravenous infusion, starting 15-30 minutes prior to standard fraction radiation therapy (1.8-2.0 Gy). (2.2)
DOSAGE FORMS AND STRENGTHS
- For injection: sterile lyophilized powder in 10 mL single-dose vials.
- Each vial contains 500 mg of amifostine on the anhydrous basis.
CONTRAINDICATIONS
ETHYOL is contraindicated in patients with known hypersensitivity to aminothiol compounds. (4)
WARNINGS AND PRECAUTIONS
- Hypotension and Cardiovascular Events: Patients who are hypotensive or dehydrated should not receive ETHYOL. If interruption of antihypertensive therapy is possible, interrupt antihypertensive therapy 24 hours prior to ETHYOL administration. Monitor blood pressure during infusion; interrupt and restart infusion if decrease in systolic blood pressure is observed. Do not administer ETHYOL to hypotensive patients who are taking antihypertensive therapy that cannot be stopped for 24 hours prior to ETHYOL administration. (5.3)
- Severe Cutaneous Reactions: Monitor patients prior to, during, and weeks after administration for severe cutaneous reactions. Discontinue for cutaneous reactions or lesions appearing outside of the injection site/radiation port or on the palms or soles. (5.4)
- Hypersensitivity: Discontinue for severe acute allergic reactions. Administer treatment for serious allergic events. (5.5)
- Nausea and Vomiting: Administer antiemetic medication prior to and in conjunction with ETHYOL. Monitor fluid balance when administered with highly emetogenic chemotherapy. (5.6)
- Hypocalcemia: Monitor serum calcium levels in patients at risk of hypocalcemia. If necessary, administer calcium supplements. (5.7)
- Embryo-Fetal Toxicity: ETHYOL can cause fetal harm. Advise patients of the potential risk to a fetus (5.8, 8.1, 8.3). Also, refer to the cisplatin full prescribing information for pregnancy and contraception information.
ADVERSE REACTIONS
Most common adverse reactions are hypotension, nausea and vomiting. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Clinigen, Inc. at 1-877-776-5385 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
- Closely monitor patients receiving anti-hypertensive medications or other drugs that could cause or potentiate hypotension. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Reduction of Cumulative Renal Toxicity with Chemotherapy
1.2 Reduction of Moderate to Severe Xerostomia from Radiation of the Head and Neck
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dose for Reduction of Cumulative Renal Toxicity with Chemotherapy
2.3 Recommended Dose for Reduction of Moderate to Severe Xerostomia from Radiation of the Head and Neck
2.4 Dose Modifications for Infusion-Related Reactions
2.5 Preparation
2.6 Incompatibilities
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Effectiveness of the Chemotherapy Regimen
5.2 Effectiveness of Radiotherapy
5.3 Hypotension and Cardiovascular Events
5.4 Severe Cutaneous Reactions
5.5 Hypersensitivity
5.6 Nausea and Vomiting
5.7 Hypocalcemia
5.8 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Reduction of Cumulative Renal Toxicity with Chemotherapy
14.2 Reduction of Moderate to Severe Xerostomia from Radiation of the Head and Neck
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Reduction of Cumulative Renal Toxicity with Chemotherapy
ETHYOL (amifostine) is indicated to reduce the cumulative renal toxicity associated with repeated administration of cisplatin in patients with advanced ovarian cancer [see Clinical Studies (14.1)].
1.2 Reduction of Moderate to Severe Xerostomia from Radiation of the Head and Neck
ETHYOL is indicated to reduce the incidence of moderate to severe xerostomia in patients undergoing post-operative radiation treatment for head and neck cancer, where the radiation port includes a substantial portion of the parotid glands [see Clinical Studies (14.2)].
Limitation of Use
Do not use ETHYOL in other settings where chemotherapy can produce a significant survival benefit or cure [see Warnings and Precautions (5.1)], or in patients receiving definitive radiotherapy [see Warnings and Precautions (5.2)], except in the context of a clinical study.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Hydration and Premedication
Prior to ETHYOL infusion, verify that patients are adequately hydrated and correct existing dehydration if clinically indicated.When administering ETHYOL at the 910 mg/m2 dose, antiemetic medications, including intravenous dexamethasone 20 mg and a serotonin 5HT3 receptor antagonist, are recommended prior to ETHYOL administration. Additional antiemetics may be required based on the chemotherapy drugs administered.
When administering ETHYOL at the 200 mg/m2 dose, it is recommended that antiemetic medication be administered prior to ETHYOL administration. Oral 5HT3 receptor antagonists, alone or in combination with other antiemetics are recommended in the radiotherapy setting.
Supine Position and Blood Pressure Monitoring
Patients should be kept in a supine position during the ETHYOL infusion.When administering ETHYOL at the 910 mg/m2 dose, monitor blood pressure before, at least every 5 minutes during the infusion, at the end of the infusion, and thereafter as clinically indicated.
When administering ETHYOL at the 200 mg/m2 dose, monitor blood pressure before and at the end of the infusion, and thereafter as clinically indicated.
2.2 Recommended Dose for Reduction of Cumulative Renal Toxicity with Chemotherapy
The recommended starting dose of ETHYOL is 910 mg/m2 administered as a 15-minute intravenous infusion, starting 30 minutes prior to chemotherapy. Do not exceed a 15-minute infusion time due to the increased risk of infusion-related reactions. The use of less than 15-minute infusion times for ETHYOL use with chemotherapy have not been established.
2.3 Recommended Dose for Reduction of Moderate to Severe Xerostomia from Radiation of the Head and Neck
The recommended dose of ETHYOL is 200 mg/m2 administered as a 3-minute intravenous infusion, starting 15-30 minutes prior to standard fraction radiation therapy (1.8-2.0 Gy).
2.4 Dose Modifications for Infusion-Related Reactions
The infusion of ETHYOL should be interrupted if the systolic blood pressure decreases significantly from the baseline value as listed in Table 1.
If severe infusion-related reactions occur, immediately and permanently discontinue ETHYOL.
Table 1: Interrupting ETHYOL Infusion Due to Decreases in Systolic Blood Pressure Baseline Systolic Blood Pressure (mm Hg)
<100
100-119
120-139
140-179
≥180
Decrease in systolic blood pressure during infusion of ETHYOL (mm Hg)
20
25
30
40
50
If the blood pressure returns to normal within 5 minutes and the patient is asymptomatic, the infusion may be restarted so that the full dose of ETHYOL may be administered.
When administering ETHYOL at the 910 mg/m2 dose, if the full dose of ETHYOL cannot be administered, the dose of ETHYOL for subsequent chemotherapy cycles should be 740 mg/m2.
2.5 Preparation
Reconstitution
During reconstitution of ETHYOL, the use of gloves is recommended, and avoid contact with the skin or mucous membranes. Follow special handling and disposal procedures [see References (15)].A vial of ETHYOL may contain more drug than is required for the recommended dose or multiple vials may be needed to obtain the recommended dose.
Reconstitute ETHYOL with 9.7 mL of sterile 0.9% Sodium Chloride Injection, USP. The reconstituted solution contains a concentration of 50 mg/mL amifostine, and should be colorless. The reconstituted solution is chemically stable for up to 5 hours at room temperature (approximately 25°C) or up to 24 hours under refrigeration (2°C to 8°C).
Dilution
Further dilute to ETHYOL with 0.9% Sodium Chloride Injection, USP to a final concentration of 5 mg/mL to 40 mg/mL before administration. ETHYOL prepared in polyvinylchloride (PVC) bags at concentrations ranging from 5 mg/mL to 40 mg/mL is chemically stable for up to 5 hours when stored at room temperature (approximately 25°C) or up to 24 hours when stored under refrigeration (2°C to 8°C).Parenteral products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Do not use ETHYOL if cloudiness or precipitate is observed.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Effectiveness of the Chemotherapy Regimen
ETHYOL may interfere with the antitumor activity of chemotherapy regimens. Do not use ETHYOL in patients receiving chemotherapy for other malignancies in which chemotherapy can produce a significant survival benefit or cure (e.g., certain malignancies of germ cell origin), except in the context of a clinical study. Limited data are currently available regarding interference with antitumor efficacy when ETHYOL is administered prior to cisplatin therapy in settings other than advanced ovarian cancer.
5.2 Effectiveness of Radiotherapy
ETHYOL may interfere with the antitumor activity of the radiotherapy regimens. Do not use ETHYOL in patients receiving definitive radiotherapy, except in the context of a clinical trial, since there are insufficient data to exclude a tumor-protective effect in this setting. ETHYOL was studied with standard fractionated radiotherapy and when ≥75% of both parotid glands were exposed to radiation. The safety and efficacy of ETHYOL on the incidence of xerostomia in the setting of combined chemotherapy and radiotherapy, and in the setting of accelerated or hyperfractionated therapy, have not been established.
5.3 Hypotension and Cardiovascular Events
Severe hypotension with serious sequelae have been reported in clinical studies and post-marketing experience in patients treated with ETHYOL. Severe hypotension events included apnea, dyspnea, hypoxia, chest pain, tachycardia, bradycardia, extrasystoles, supraventricular tachycardia, atrial fibrillation/flutter, myocardial ischemia, myocardial infarction, unconsciousness, syncope, seizure, renal failure, and respiratory and cardiac arrest.
In the WR-1 study of patients with ovarian cancer dosing ETHYOL at 910 mg/m2, transient hypotension was observed in 62% of the patients treated, with 8% of the patients experiencing > Grade 3 hypotension. The mean time of onset was 14 minutes after initiation of the ETHYOL infusion, the mean duration of hypotension was 6 minutes, and blood pressure returned to normal within 15 minutes after the onset of hypotension in most cases. Approximately 3% of patients permanently discontinued ETHYOL due to severe hypotension.
In the WR-38 study of patients with head and neck cancer administering ETHYOL at a dose of 200 mg/m2 prior to radiotherapy, hypotension was observed in 15% of patients treated, with 3% of the patients experiencing > Grade 3 hypotension.
Before administration of ETHYOL, verify that patients are not hypotensive or dehydrated. Adequately hydrate patients prior to initiating ETHYOL infusion.
Patients receiving ETHYOL at doses recommended for chemotherapy should have antihypertensive therapy interrupted 24 hours preceding administration of ETHYOL. Patients receiving ETHYOL at doses recommended for chemotherapy who are taking antihypertensive therapy that cannot be stopped for 24 hours preceding ETHYOL treatment should avoid treatment with ETHYOL. During and after ETHYOL infusion, closely monitor the blood pressure of patients whose antihypertensive medication has been interrupted since hypertension may be exacerbated by discontinuation of antihypertensive medication or other causes such as intravenous hydration.
During ETHYOL infusion, keep patients in a supine position and monitor blood pressure every 5 minutes during the infusion, and thereafter as clinically indicated. For infusion durations less than 5 minutes, blood pressure should be monitored at least before and immediately after the infusion, and thereafter as clinically indicated.
It is important that the duration of the 910 mg/m2 infusion does not exceed 15 minutes, as administration of ETHYOL as a longer infusion is associated with a higher incidence of adverse reactions.
If hypotension occurs, place patients in the Trendelenburg position and give an infusion of normal saline using a separate intravenous line. If the blood pressure returns to normal within 5 minutes and the patient is asymptomatic, the infusion may be restarted, so that the full dose of ETHYOL can be administered. Guidelines for interrupting and restarting ETHYOL infusion if a decrease in systolic blood pressure should occur are provided [see Dosage and Administration (2.1)].
5.4 Severe Cutaneous Reactions
Fatal and severe cutaneous reactions have been reported in clinical studies and post-marketing experience in patients treated with ETHYOL. Severe cutaneous reactions include erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, toxicoderma, exfoliative dermatitis and drug reaction with biopsy‑confirmed eosinophilia and systemic symptoms (DRESS).
These reactions have been reported more frequently when ETHYOL is used as a radioprotectant [see Adverse Reactions (6)]. Severe cutaneous reactions may develop weeks after initiation of ETHYOL administration. Monitor patients carefully prior to, during, and after ETHYOL administration.
Discontinue ETHYOL for cutaneous reactions or mucosal lesions appearing outside of the injection site or radiation port and for erythematous, edematous or bullous lesions on the palms or soles.
5.5 Hypersensitivity
Hypersensitivity reactions, including anaphylaxis, have been reported in clinical studies and post-marketing experience with ETHYOL administration. Hypersensitivity and anaphylactic reactions observed during or after ETHYOL administration have included pyrexia, chills, dyspnea, hypoxia, chest discomfort, cutaneous eruptions, pruritus, urticaria, and laryngeal edema.
Epinephrine and other appropriate measures should be available for treatment of serious infusion-related reactions when administering ETHYOL. When severe allergic reactions occur, immediately and permanently discontinue ETHYOL.
5.6 Nausea and Vomiting
Nausea and/or vomiting occur frequently after ETHYOL infusion and may be severe. In the WR-1 study of patients with ovarian cancer dosing ETHYOL at 910 mg/m2, vomiting was observed in 96% of the patients treated, with severe nausea/vomiting on day 1 of cyclophosphamide-cisplatin chemotherapy in 19% of patients.
In the WR-38 study of patients with head and neck cancer administering ETHYOL at a dose of 200 mg/m2 prior to radiotherapy, vomiting was observed in 53% of patients treated, with severe nausea/vomiting in 8% of patients.
Administer antiemetic medication(s) prior to and in conjunction with ETHYOL [see Dosage and Administration (2.1)]. When ETHYOL is administered with highly emetogenic chemotherapy, closely monitor the fluid balance of the patient.
5.7 Hypocalcemia
Monitor serum calcium levels in patients at risk of hypocalcemia, such as those with nephrotic syndrome, or patients receiving multiple doses of ETHYOL.
At the recommended doses, clinically significant hypocalcemia was reported in 1% of patients in the head and neck cancer study (WR-38).
If necessary, calcium supplements can be administered.
5.8 Embryo-Fetal Toxicity
Based on findings in animals, ETHYOL can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, intravenous administration of ETHYOL to pregnant rabbits during organogenesis was embryotoxic at doses approximately sixty percent of the recommended dose in humans based on body surface area. Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
When ETHYOL is used in combination with cisplatin, refer to the cisplatin full prescribing information for pregnancy and contraception information.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
ETHYOL was administered to patients receiving chemotherapeutic agents for advanced ovarian cancer (WR-1 study) or who were receiving standard fractionated radiotherapy for head and neck cancer (WR-38 study) [see Clinical Studies (14)].
In the WR-38 study of patients with head and neck cancer, 17% (26/150) discontinued ETHYOL due to adverse reactions. All but one of these patients continued to receive radiation treatment until completion.
Table 2 summarizes adverse reactions reported in patients from the WR-1 and WR-38 clinical trials.
Table 2: Incidence of Common Adverse Reactions in Patients Receiving ETHYOL Ovarian Cancer
(WR-1 Trial)
910 mg/m2_____________________________________________
Per Patient Per Infusion
Head and Neck Cancer
(WR-38 Trial)
200 mg/m2
_______________________________________________Per Patient Per Infusion
Nausea/Vomiting
≥Grade 3
All Grades
36/122 (30%)
117/122 (96%)
53/592 (9%)
520/592 (88%)
12/150 (8%)
80/150 (53%)
13/4314 (<1%)
233/4314 (5%)Hypotension
≥Grade 3
All Grades
10/122 (8%)
75/122 (62%)
159/592 (27%)
4/150 (3%)
22/150 (15%)
46/4314 (1%)Other clinically relevant adverse reactions reported in patients in the WR-1 and WR-38 trials include the following:
Infusion-related Reactions: flushing/feeling of warmth, chills/feeling of coldness, malaise, pyrexia, rash, dizziness, somnolence, hiccups, diarrhea, sneezing, diplopia and blurred vision. These effects have not generally precluded the completion of therapy.
Injection site reactions (including rash/erythema, pruritus, urticaria, pain, inflammation, bruising and local swelling) were also observed.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of ETHYOL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following reported post-marketing adverse reactions are described elsewhere in the labeling:
- Hypotension and Cardiovascular Events [see Warnings and Precautions (5.3)]
- Severe Cutaneous Reactions [see Warnings and Precautions (5.4)]
- Hypersensitivity [see Warnings and Precautions (5.5)]
Adverse reactions associated with the use of ETHYOL that have been identified in other clinical trials and/or post-marketing reports are described below:
Immune system disorders: Hypersensitivity reactions including pruritus, urticaria, laryngeal edema, anaphylactic reactions, anaphylactoid reactions.
Nervous system disorders: Seizure.
Cardiac disorders: Myocardial ischemia, myocardial infarction, cardiac arrest, arrhythmias including tachycardia, bradycardia, atrial fibrillation/flutter, supraventricular tachycardia, extrasystoles.
Vascular disorders: Transient hypertension and exacerbations of preexisting hypertension.
Respiratory, thoracic and mediastinal disorders: Apnea, dyspnea, hypoxia, respiratory arrest.
Skin and subcutaneous tissue disorders: Erythema multiforme, dermatitis exfoliative, Stevens-Johnson syndrome, toxic epidermal necrolysis.
Renal and urinary disorders: Renal failure.
General disorders and administration site conditions: Chest discomfort and chest pain.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
When ETHYOL is used in combination with cisplatin, refer to the cisplatin full prescribing information for pregnancy information.
Based on findings in animals, ETHYOL can cause fetal harm when administered to a pregnant woman. There are no available data on ETHYOL use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. In animal reproduction studies, intravenous administration of ETHYOL to pregnant rabbits during organogenesis was embryotoxic at doses approximately sixty percent of the recommended dose in humans based on body surface area (see Data). Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the estimated background risk of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies in the U.S. general population.
8.2 Lactation
Risk Summary
When ETHYOL is used in combination with cisplatin, refer to the cisplatin full prescribing information for lactation information.
There are no data on the presence of amifostine or its metabolites in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise lactating women not to breastfeed during treatment with ETHYOL.
8.3 Females and Males of Reproductive Potential
When ETHYOL is used in combination with cisplatin, refer to the cisplatin full prescribing information for contraception and infertility information.
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating ETHYOL.
Infertility
Males
Based on findings from animal studies, ETHYOL may impair fertility in males of reproductive potential [see Nonclinical Toxicology (13.1)].8.5 Geriatric Use
The clinical studies did not include sufficient number of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy in elderly patients.
-
10 OVERDOSAGE
In clinical trials, the maximum single dose of ETHYOL was 1300 mg/m2. No information is available on single doses higher than this in adults. At the higher doses, anxiety and reversible urinary retention occurred.
The most likely symptom of overdosage is hypotension, which should be managed by infusion of normal saline and other supportive measures, as clinically indicated [see Warnings and Precautions (5.3)].
-
11 DESCRIPTION
ETHYOL (amifostine) is an organic thiophosphate cytoprotective agent known chemically as 2-[(3-aminopropyl)amino]ethanethiol dihydrogen phosphate (ester) and has the following structural formula:
H2N(CH2)3NH(CH2)2S-PO3H2
Amifostine is a white crystalline powder which is freely soluble in water. Its empirical formula is C5H15N2O3PS and it has a molecular weight of 214.22.
ETHYOL is the trihydrate form of amifostine and is supplied as a sterile lyophilized powder requiring reconstitution for intravenous infusion. Each single-dose 10 mL vial contains 500 mg of amifostine on the anhydrous basis.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ETHYOL is a prodrug that is dephosphorylated by alkaline phosphatase in tissues to a pharmacologically active free thiol metabolite. This metabolite is believed to be responsible for the reduction of the cumulative renal toxicity of cisplatin and for the reduction of the toxic effects of radiation on normal oral tissues. The ability of ETHYOL to differentially protect normal tissues is attributed to the higher capillary alkaline phosphatase activity, higher pH and better vascularity of normal tissues relative to tumor tissue, which results in a more rapid generation of the active thiol metabolite as well as a higher rate constant for uptake into cells. The higher concentration of the thiol metabolite in normal tissues is available to bind to, and thereby detoxify, reactive metabolites of cisplatin. This thiol metabolite can also scavenge reactive oxygen species generated by exposure to either cisplatin or radiation.
12.3 Pharmacokinetics
Clinical pharmacokinetic studies show that ETHYOL is rapidly cleared from the plasma with a distribution half-life of < 1 minute and an elimination half-life of approximately 8 minutes. Less than 10% of ETHYOL remains in the plasma 6 minutes after drug administration. ETHYOL is rapidly metabolized to an active free thiol metabolite. A disulfide metabolite is produced subsequently and is less active than the free thiol. After a 10-second bolus dose of 150 mg/m2 of ETHYOL, renal excretion of the parent drug and its two metabolites was low during the hour following drug administration, averaging 0.69%, 2.64% and 2.22% of the administered dose for the parent, thiol and disulfide, respectively. Measurable levels of the free thiol metabolite have been found in bone marrow cells 5-8 minutes after intravenous infusion of ETHYOL. Pretreatment with dexamethasone or metoclopramide has no effect on ETHYOL pharmacokinetics.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with ETHYOL.
ETHYOL was negative in the Ames test and in the mouse micronucleus test. The free thiol metabolite was positive in the Ames test with S9 microsomal fraction in the TA1535 Salmonella typhimurium strain and at the TK locus in the mouse L5178Y cell assay. The metabolite was negative in the mouse micronucleus test and negative for clastogenicity in human lymphocytes.
Fertility studies in animals have not been conducted with amifostine. In a 3-month repeat-dose toxicity study in rats, intravenous administration of amifostine resulted in bilateral degeneration of the germinal epithelium of the testes and bilateral hypospermia in the epididymides at doses of 50 mg/kg/day (approximately 0.3 times the maximum clinical dose based on body surface area).
-
14 CLINICAL STUDIES
14.1 Reduction of Cumulative Renal Toxicity with Chemotherapy
A randomized controlled trial (WR-1) compared six cycles of cyclophosphamide 1000 mg/m2, and cisplatin 100 mg/m2 with or without ETHYOL pretreatment at 910 mg/m2, in two successive cohorts with a total of 242 patients with advanced ovarian cancer.
In both cohorts, after multiple cycles of chemotherapy, pretreatment with ETHYOL significantly reduced the cumulative renal toxicity associated with cisplatin, as assessed by the proportion of patients who had ≥40% decrease in creatinine clearance from pretreatment values, protracted elevations in serum creatinine (>1.5 mg/dL), or severe hypomagnesemia.
Table 3: Patients with ≥40% Reduction in Calculated Creatinine Clearance* - * Creatinine clearance values were calculated using the Cockcroft-Gault formula.
ETHYOL+ CP CP p-value
(2-sided)All Patients 16/122 (13%) 36/120 (30%) 0.001 First Cohort 10/63 20/58 0.018 Second Cohort 6/59 16/62 0.026 Table 4: Patients with Increasing Grades of Hypomagnesemia - * NCI toxicity grades of serum magnesium levels for each patient's last cycle of therapy.
- † Based on 2-sided Mantel-Haenszel Chi-Square statistic.
NCI-CTC Grade*:
(mEq/L)0
>1.41
≤1.4->1.12
≤1.1->0.83
≤0.8->0.54
≤0.5p-value† All Patients
ETHYOL+CP
CP92
7313
183
70
50
10.001 First Cohort
ETHYOL+CP
CP49
3510
83
60
30
10.017 Second Cohort
ETHYOL+CP
CP43
383
100
10
20
00.012 Exploratory subgroup analyses suggested that the effect of ETHYOL was consistent in patients with increased risks for nephrotoxicity (i.e., nephrotoxic antibiotics, preexisting diabetes, or hypertension) compared to patients who lacked these risks. The effects of ETHYOL in reducing the cumulative renal toxicity of cisplatin in the randomized ovarian cancer study are shown in Tables 3 and 4.
In the randomized ovarian cancer study, ETHYOL had no detectable effect on the antitumor efficacy of cisplatin-cyclophosphamide chemotherapy. Objective response rates (including pathologically confirmed complete remission rates), time to progression, and survival duration were all similar in the ETHYOL and control study groups. Table 5 summarizes the efficacy findings of the WR-1 ovarian cancer study.
Table 5: Efficacy Results from the WR-1 Study ETHYOL + CP CP Complete pathologic tumor
response rate21.3% 15.8% Time to progression (months) Median (± 95% CI) 15.8 (13.2, 25.1) 18.1 (12.5, 20.4) Mean (± Std error) 19.8 (±1.04) 19.1 (±1.58) Hazard ratio
(95% Confidence Interval).98 (.64, 1.4) Survival (months) Median (± 95% CI) 31.3 (28.3, 38.2) 31.8 (26.3, 39.8) Mean (± Std error) 33.7 (±2.03) 34.3 (±2.04) Hazard ratio
(95% Confidence Interval).97 (.69, 1.32) 14.2 Reduction of Moderate to Severe Xerostomia from Radiation of the Head and Neck
A randomized controlled trial (WR-38) of standard fractionated radiation (1.8 Gy - 2.0 Gy/day for 5 days/week for 5-7 weeks) with or without ETHYOL, administered at 200 mg/m2 as a 3 minute intravenous infusion 15‑30 minutes prior to each fraction of radiation, was conducted in 315 patients with head and neck cancer. Patients were required to have at least 75% of both parotid glands in the radiation field.
The incidence of Grade 2 or higher acute (90 days or less from start of radiation) and late xerostomia (9‑12 months following radiation) as assessed by RTOG Acute and Late Morbidity Scoring Criteria, was significantly reduced in patients receiving ETHYOL (Table 6).
Table 6: Incidence of Grade 2 or Higher Xerostomia (RTOG criteria) - * Based on the number of patients for whom actual data were available.
ETHYOL + RT RT p-value Acute
(≤90 days from
start of radiation)51% (75/148) 78% (120/153) p<0.0001 Late*
(9-12 months
post radiation)35% (36/103) 57% (63/111) p=0.0016 At one year following radiation, whole saliva collection following radiation showed that more patients given ETHYOL produced >0.1 gm of saliva (72% vs. 49%). In addition, the median saliva production at one year was higher in those patients who received ETHYOL (0.26 gm vs. 0.1 gm). Stimulated saliva collections did not show a difference between treatment arms. These improvements in saliva production were supported by the patients' subjective responses to a questionnaire regarding oral dryness.
In the randomized head and neck cancer study, locoregional control, disease-free survival and overall survival were all comparable in the two treatment groups after one year of follow-up (see Table 7).
Table 7: Efficacy Results at 1 Year from the WR-38 Study - * 1 year rates estimated using Kaplan-Meier method
- † Hazard ratio >1.0 is in favor of the ETHYOL + RT arm
ETHYOL + RT RT Locoregional Control Rate* 76.1% 75.0% Hazard Ratio† 1.013 95% Confidence Interval (0.671, 1.530) Disease-Free Survival Rate* 74.6% 70.4% Hazard Ratio† 1.035 95% Confidence Interval (0.702, 1.528) Overall Survival Rate* 89.4% 82.4% Hazard Ratio† 1.585 95% Confidence Interval (0.961, 2.613) - 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
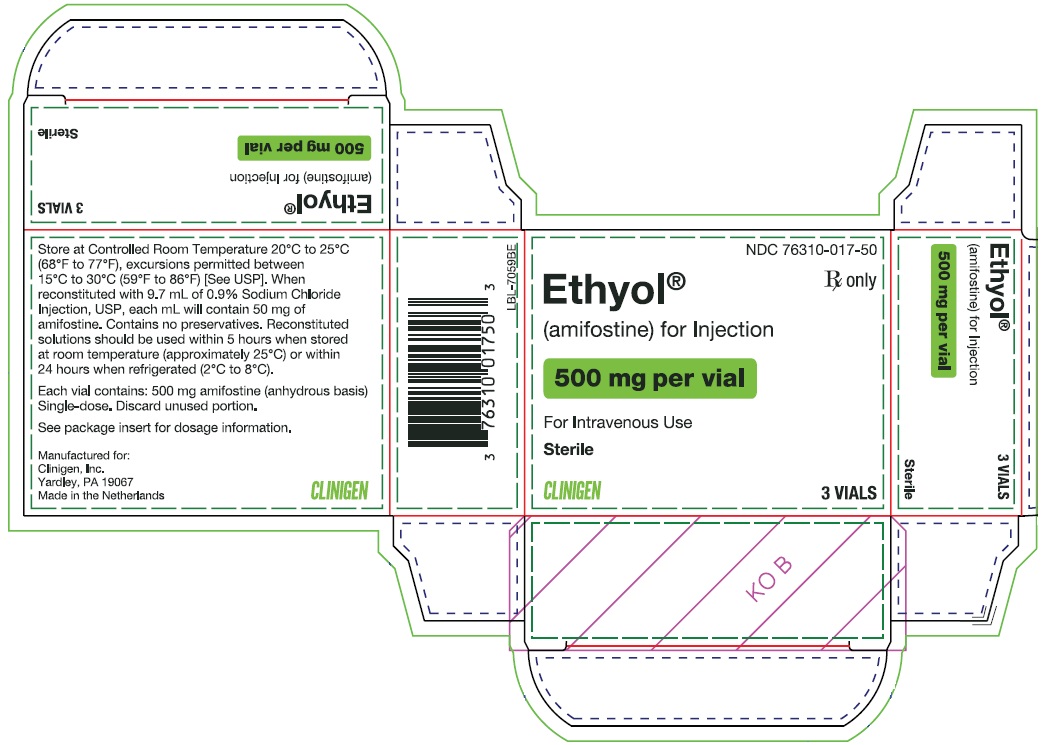
ETHYOL (amifostine) for Injection is supplied as a sterile lyophilized white powder in 10 mL single-dose vials (NDC: 76310-017-50). Each vial contains 500 mg of amifostine on the anhydrous basis. The vials are available packaged as follows:
Carton containing 3 vials (NDC: 76310-017-50)
Discard unused portion.
Store the lyophilized dosage form at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
Follow special handling and disposal procedures [see References (15)].
-
17 PATIENT COUNSELING INFORMATION
Hypotension and Cardiovascular Events
Inform the patient that he or she may experience hypotension after ETHYOL infusion [see Adverse Reactions (6.1)]. Advise the patient to inform the prescriber if he or she is receiving anti-hypertensive medications or other drugs that could cause or potentiate hypotension [see Warnings and Precautions (5.3), Drug Interactions (7)].Severe Cutaneous Reactions
Inform the patient that severe cutaneous reactions may develop during or weeks after initiation of ETHYOL administration. Advise the patient to inform the prescriber if they experience any cutaneous reactions or mucosal lesions outside of the injection site or radiation port, or on the palms or soles, as discontinuation of the drug may be necessary [see Warnings and Precautions (5.4)].Hypersensitivity
Inform the patient that he or she may experience allergic reactions after ETHYOL infusion [see Adverse Reactions (6.1)]. ETHYOL might need to be discontinued for severe acute allergic reactions and the patient receive other treatments [See Warnings and Precautions (5.5)].Nausea and Vomiting
Inform the patient that he or she may experience severe nausea and/or vomiting after ETHYOL infusion [see Adverse Reactions (6.1)].Hypocalcemia
Inform the patient that calcium supplements may be necessary if hypocalcemia is identified [see Warnings and Precautions (5.7)]. Blood tests may be needed to identify this, and correct the condition.Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.8) and Use in Specific Populations (8.1)]. Refer to the cisplatin full prescribing information for pregnancy and contraception information.Lactation
Advise lactating women not to breastfed during treatment with ETHYOL [see Use in Specific Populations (8.2)]. Refer to the cisplatin full prescribing information for lactation information.Infertility
Advise males of reproductive potential that ETHYOL may impair fertility [see Use in Specific Populations (8.3)]. Refer to the cisplatin full prescribing information for infertility information.U.S. Patent 5,994,409
Manufactured for:
Clinigen, Inc.
Yardley, PA 19067ETHYOL is a registered trademark owned by the Clinigen Group.
For additional information, contact Clinigen, Inc. 1-877-776-5385.
Revision Date: 12/2019
- VIAL PRINCIPAL DISPLAY PANEL - 500 mg
- CARTON PRINCIPAL DISPLAY PANEL - 500 mg
-
INGREDIENTS AND APPEARANCE
ETHYOL
amifostine injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 76310-017 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMIFOSTINE (UNII: M487QF2F4V) (AMIFOSTINE ANHYDROUS - UNII:ILA426L95O) AMIFOSTINE ANHYDROUS 500 mg in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76310-017-50 3 in 1 CARTON 01/01/2020 1 NDC: 76310-017-01 10 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020221 01/01/2020 Labeler - Clinigen Group PLC (211471076)
Trademark Results [Ethyol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ETHYOL 76339868 2585403 Dead/Cancelled |
CLINIGEN GROUP PLC 2001-11-20 |
 ETHYOL 73751893 1618361 Live/Registered |
U.S. BIOSCIENCE, INC. 1988-09-14 |
 ETHYOL 73722551 not registered Dead/Abandoned |
U.S. BIOSCIENCE, INC. 1988-04-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.