STAY AWAKE MAXIMUM STRENGTH- caffeine tablet
Stay Awake by
Drug Labeling and Warnings
Stay Awake by is a Otc medication manufactured, distributed, or labeled by Better Living Brands, LLC, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
For occasional use only
Caffeine warning: The recommended dose of this product contains about as muchcaffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
Signature™
care
Quality Guaranteed
COMPARE TO
Vivarin®
active ingredient*NDC: 21130-226-10
Maximum Strength
Stay AwakeCAFFEINE 200 mg
Alertness AidEqual to about a cup of coffee
Actual
Size40 TABLETS
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING*This product is not manufactured or distributed
by Meda AB, distributors of Vivarin®.
50844 REV1219B22610DISTRIBUTED BY
BETTER LIVING BRANDS LLC
P.O. BOX 99, PLEASANTON, CA 94566-0009
1-888-723-3929
www.betterlivingbrandsLLC.comOUR PROMISE
QUALITY & SATISFACTION
100%
GUARANTEED
OR YOUR MONEY BACK.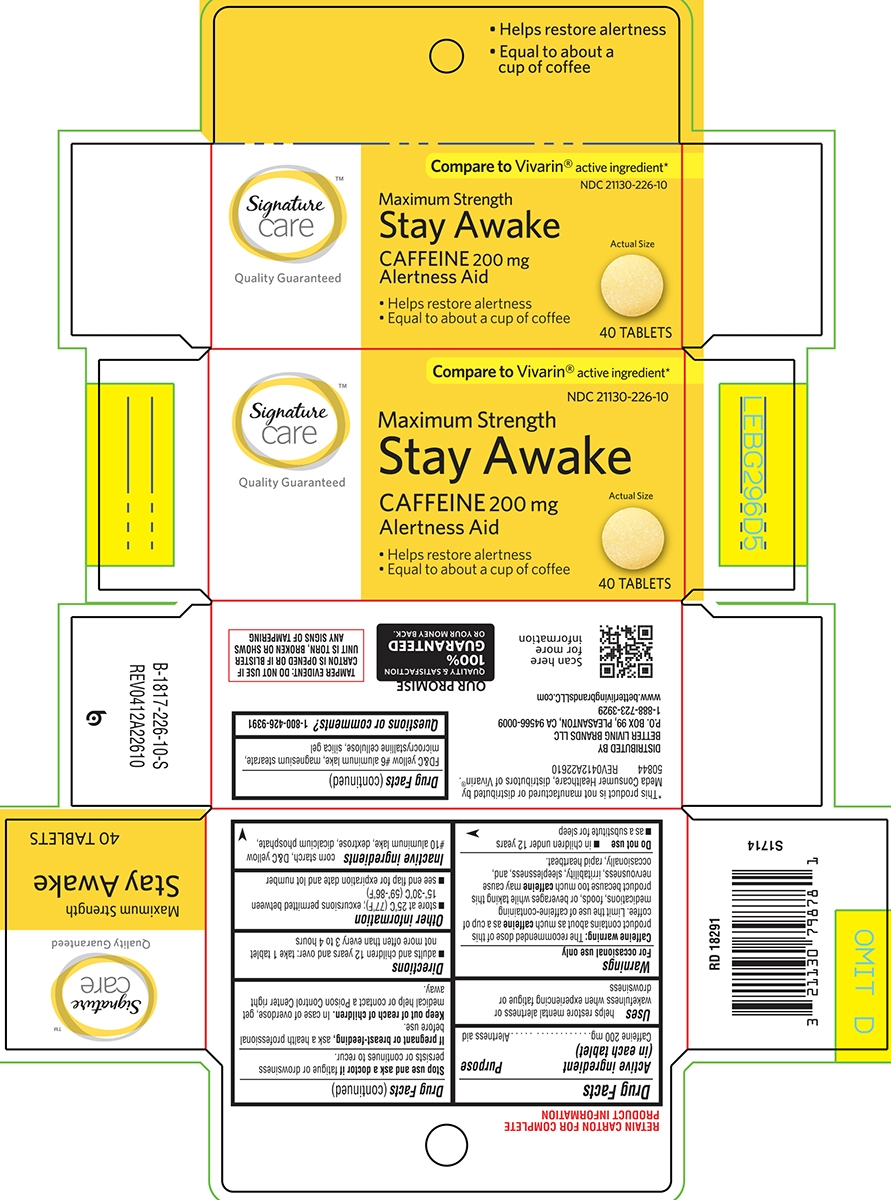
Signature Care 44-226
-
INGREDIENTS AND APPEARANCE
STAY AWAKE MAXIMUM STRENGTH
caffeine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 21130-226 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 200 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color YELLOW Score no score Shape ROUND Size 11mm Flavor Imprint Code 44;226 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 21130-226-10 5 in 1 CARTON 11/21/1996 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part340 11/21/1996 Labeler - Better Living Brands, LLC (009137209) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(21130-226) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(21130-226) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(21130-226) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(21130-226) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(21130-226)
Trademark Results [Stay Awake]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 STAY AWAKE 85047007 not registered Dead/Abandoned |
A.N.N.A. LLC 2010-05-25 |
 STAY AWAKE 76575252 not registered Dead/Abandoned |
Pharmaceutical Formulations, Inc. 2004-02-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.