VETROPOLYCIN HC- bacitracin zinc, neomycin sulfate, polymyxin b sulfate, and hydrocortisone acetate ointment
Vetropolycin HC by
Drug Labeling and Warnings
Vetropolycin HC by is a Animal medication manufactured, distributed, or labeled by Dechra Veterinary Products, Altaire Pharmaceuticals, Inc., Xellia Pharmaceuticals Inc., Pharmacia and Upjohn Company, Xellia Pharmaceuticals AS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
- VETERINARY INDICATIONS
- DOSAGE & ADMINISTRATION
- CONTRAINDICATIONS
-
WARNINGS
WARNINGS: All topical ophthalmic preparations containing corticosteroids with or without an antimicrobial agent, are contraindicated in the initial treatment of corneal ulcers. They should not be used until the infection is under control and corneal regeneration is well under way.
Serious hypersensitivity (anaphylactic) reactions have been reported in cats within 4 hours of application of antibiotic ophthalmic preparations.
Some of these reactions have resulted in death.
Clinical and experimental data have demonstrated that corticosteroids administered orally or by injection to animals may induce the first stage of parturition if used during the last trimester of pregnancy and may precipitate premature parturition followed by dystocia, fetal death, retained placenta, and metritis.
Additionally, corticosteroids administered to dogs, rabbits, and rodents during pregnancy have resulted in cleft palate in offspring.
Corticosteroids administered to dogs during pregnancy have also resulted in other congenital anomalies, including deformed forelegs, phocomelia, and anasarca.
- SAFE HANDLING WARNING
-
PRECAUTIONS
PRECAUTIONS: Sensitivity to this ophthalmic ointment is rare, however, if a reaction occurs, discontinue use of the preparation. The prolonged use of antibiotic-containing preparations may result in overgrowth of nonsusceptible organisms including fungi. Appropriate measures should be taken if this occurs.
If infection does not respond to treatment in two or three days, the diagnosis and therapy should be re-evaluated.
Animals under treatment with this product should be observed for usual signs of corticosteroid overdose which include polydipsia, polyuria and occasionally an increase in weight. Use of corticosteroids, depending on dose, duration, and specific steroid, may result in inhibition of endogenous steroid production following drug withdrawal. In patients presently receiving or recently withdrawn from systemic corticosteroid treatments, therapy with a rapidly acting corticosteroid should be considered in unusually stressful situations. Care should be taken not to contaminate the applicator tip during the administration of the preparation.
-
ADVERSE REACTIONS
ADVERSE REACTIONS: Itching, burning or inflammation may occur in animals sensitive to the product. Discontinue use in such cases.
SAP and SGPT (ALT) enzyme elevations, polydipsia and polyuria have occurred following parenteral or systemic use of synthetic corticosteroids in dogs. Vomiting and diarrhea (occasionally bloody) have been observed in dogs. Cushing's syndrome in dogs has been reported in association with prolonged or repeated steroid therapy.
For a copy of the Safety Data Sheet (SDS), or to report adverse reactions, call Dechra Veterinary Products at (866) 933-2472.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or http://www.fda.gov/AnimalVeterinary/SafetyHealth
-
MECHANISM OF ACTION
ACTIONS: The overlapping spectra of these three antibiotics provide effective bactericidal action against most commonly occurring gram-positive and gram-negative bacteria associated with infections of the eyes. The range of bactericidal activity encompasses many bacteria which are, or have become, resistant to other antibiotics, notably Pseudomonas and Staphylococcus.
In susceptible organisms, resistance rarely develops, even on repeated or prolonged usage. Hydrocortisone acetate exerts a marked anti-inflammatory action at the tissue level and effectively suppresses inflammation in many disorders of the anterior segment of the eye. Local application to the eye often gives rapid relief of pain and photophobia, particularly in lesions of the cornea. The combined anti-inflammatory and antimicrobial activity of Vetropolycin HC (neomycin and polymyxin B sulfates, bacitracin zinc, and hydrocortisone acetate ophthalmic ointment) Veterinary Ophthalmic Ointment permits effective management of many disorders of the anterior segment of the eye in which combined activity is needed.
- STORAGE AND HANDLING
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
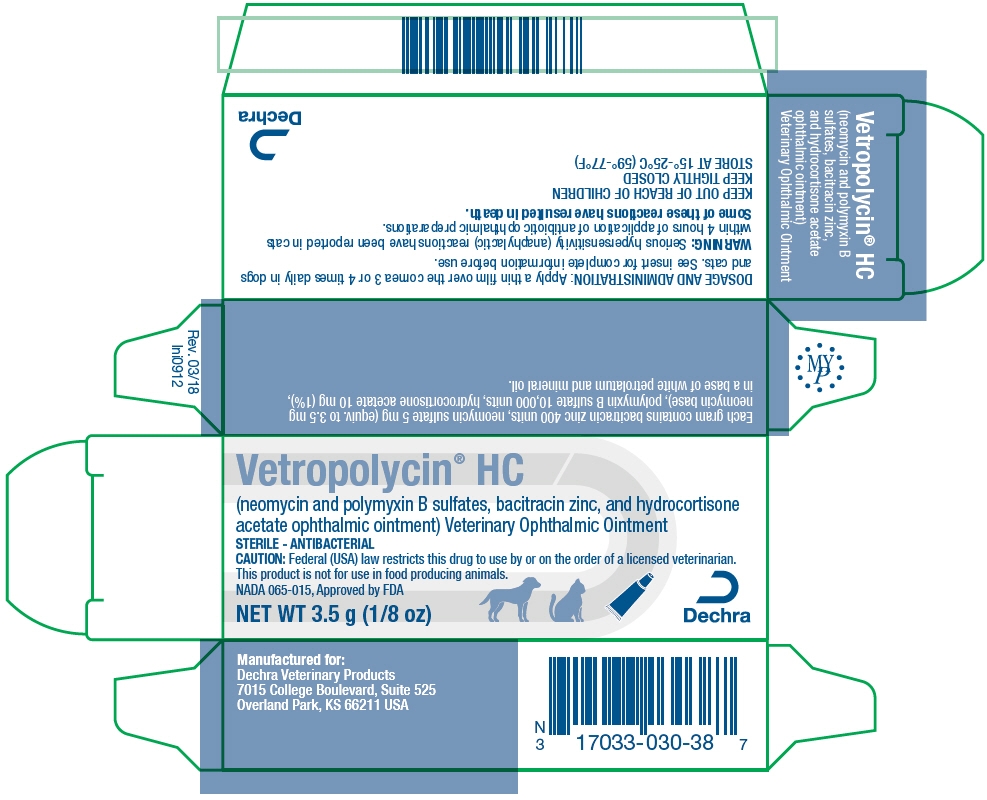
PRINCIPAL DISPLAY PANEL - 3.5 g Tube Carton
Vetropolycin® HC
(neomycin and polymyxin B sulfates, bacitracin zinc, and hydrocortisone
acetate ophthalmic ointment) Veterinary Ophthalmic OintmentSTERILE - ANTIBACTERIAL
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
This product is not for use in food producing animals.
NADA 065-015, Approved by FDANET WT 3.5 g (1/8 oz)
Dechra
-
INGREDIENTS AND APPEARANCE
VETROPOLYCIN HC
bacitracin zinc, neomycin sulfate, polymyxin b sulfate, and hydrocortisone acetate ointmentProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 17033-030 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Bacitracin Zinc (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [USP'U] in 1 g Neomycin Sulfate (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 5 mg in 1 g Polymyxin B Sulfate (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [USP'U] in 1 g Hydrocortisone Acetate (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) Hydrocortisone Acetate 10 mg in 1 g Inactive Ingredients Ingredient Name Strength mineral oil (UNII: T5L8T28FGP) petrolatum (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17033-030-38 1 in 1 CARTON 1 3.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA065015 04/10/2014 Labeler - Dechra Veterinary Products (362142734) Establishment Name Address ID/FEI Business Operations Altaire Pharmaceuticals, Inc. 786790378 MANUFACTURE Establishment Name Address ID/FEI Business Operations Xellia Pharmaceuticals Inc. 529171201 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Pharmacia and Upjohn Company 618054084 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Xellia Pharmaceuticals AS 305814345 API MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.