Flucytosine Capsules Rx only
Flucytosine by
Drug Labeling and Warnings
Flucytosine by is a Prescription medication manufactured, distributed, or labeled by Bryant Ranch Prepack. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FLUCYTOSINE- flucytosine capsule
Bryant Ranch Prepack
----------
Flucytosine
Capsules
Rx only
WARNING
Use with extreme caution in patients with impaired renal function. Close monitoring of hematologic, renal and hepatic status of all patients is essential. These instructions should be thoroughly reviewed before administration of Flucytosine Capsules.
DESCRIPTION
Flucytosine Capsules, an antifungal agent, are available as 250 mg and 500 mg capsules for oral administration. In addition to the active ingredient of flucytosine, each capsule contains corn starch, lactose and talc. The 250 mg capsule shell contains black iron oxide, D&C Yellow No. 10, FD&C Blue No. 1, FD&C Yellow No. 6, gelatin and titanium dioxide. The 500 mg capsule shell contains black iron oxide, gelatin and titanium dioxide.
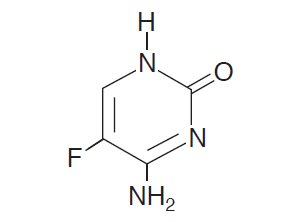
Chemically, flucytosine is 5-fluorocytosine, a fluorinated pyrimidine which is related to fluorouracil and floxuridine. It is a white to off-white crystalline powder with a molecular weight of 129.09 and the following structural formula:
CLINICAL PHARMACOLOGY
Flucytosine is rapidly and virtually completely absorbed following oral administration. Flucytosine Capsules are not metabolized significantly when given orally to man. Bioavailability estimated by comparing the area under the curve of serum concentrations after oral and intravenous administration showed 78% to 89% absorption of the oral dose. Peak serum concentrations of 30 to 40 mcg/mL were reached within 2 hours of administration of a 2 g oral dose to normal subjects. Other studies revealed mean serum concentrations of approximately 70 to 80 mcg/mL 1 to 2 hours after a dose in patients with normal renal function receiving a 6-week regimen of flucytosine (150 mg/kg/day given in divided doses every 6 hours) in combination with amphotericin B. The half-life in the majority of healthy subjects ranged between 2.4 and 4.8 hours. Flucytosine is excreted via the kidneys by means of glomerular filtration without significant tubular reabsorption. More than 90% of the total radioactivity after oral administration was recovered in the urine as intact drug. Flucytosine is deaminated (probably by gut bacteria) to 5-fluorouracil. The area under the curve (AUC) ratio of 5-fluorouracil to flucytosine is 4%. Approximately 1% of the dose is present in the urine as the α-fluoro-β-ureido-propionic acid metabolite. A small portion of the dose is excreted in the feces.
The half-life of flucytosine is prolonged in patients with renal insufficiency; the average half-life in nephrectomized or anuric patients was 85 hours (range: 29.9 to 250 hours). A linear correlation was found between the elimination rate constant of flucytosine and creatinine clearance.
In vitro studies have shown that 2.9% to 4% of flucytosine is protein-bound over the range of therapeutic concentrations found in the blood. Flucytosine readily penetrates the blood-brain barrier, achieving clinically significant concentrations in cerebrospinal fluid.
Pharmacokinetics in Pediatric Patients
Limited data are available regarding the pharmacokinetics of Flucytosine Capsules administered to neonatal patients being treated for systemic candidiasis. After five days of continuous therapy, median peak levels in infants were 19.6 mcg/mL, 27.7 mcg/mL, and 83.9 mcg/mL at doses of 25 mg/kg (N=3), 50 mg/kg (N=4), and 100 mg/kg (N=3), respectively. Mean time to peak serum levels was of 2.5 ± 1.3 hours, similar to that observed in adult patients. A good deal of interindividual variability was noted, which did not correlate with gestational age. Some patients had serum levels > 100 mcg/mL, suggesting a need for drug level monitoring during therapy. In another study, serum concentrations were determined during flucytosine therapy in two patients (total assays performed =10). Median serum flucytosine concentrations at steady state were calculated to be 57 ± 10 mcg/mL (doses of 50 to 125 mg/kg/day, normalized to 25 mg/kg per dose for comparison). In three infants receiving flucytosine 25 mg/kg/day (four divided doses), a median flucytosine half-life of 7.4 hours was observed, approximately double that seen in adult patients. The concentration of flucytosine in the cerebrospinal fluid of one infant was 43 mcg/mL 3 hours after a 25 mg oral dose, and ranged from 20 to 67 mg/L in another neonate receiving oral doses of 120 to 150 mg/kg/day.
MICROBIOLOGY
Mechanism of Action
Flucytosine is taken up by fungal organisms via the enzyme cytosine permease. Inside the fungal cell, flucytosine is rapidly converted to fluorouracil by the enzyme cytosine deaminase. Fluorouracil exerts its antifungal activity through the subsequent conversion into several active metabolites, which inhibit protein synthesis by being falsely incorporated into fungal RNA or interfere with the biosynthesis of fungal DNA through the inhibition of the enzyme thymidylate synthetase.
Activity In Vitro
Flucytosine has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections.
Candida albicans
Cryptococcus neoformans
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
Drug Resistance
Flucytosine resistance may arise from a mutation of an enzyme necessary for the cellular uptake or metabolism of flucytosine or from an increased synthesis of pyrimidines, which compete with the active metabolites of flucytosine (fluorinated antimetabolites). Resistance to flucytosine has been shown to develop during monotherapy after prolonged exposure to the drug.
Drug Combination
Antifungal synergism between flucytosine and polyene antibiotics, particularly amphotericin B has been reported in vitro. Flucytosine Capsules are usually administered in combination with amphotericin B due to lack of cross-resistance and reported synergistic activity of both drugs.
INDICATIONS AND USAGE
Flucytosine Capsules are indicated only in the treatment of serious infections caused by susceptible strains of Candida and/or Cryptococcus.
Candida: Septicemia, endocarditis and urinary system infections have been effectively treated with flucytosine. Limited trials in pulmonary infections justify the use of flucytosine
Cryptococcus: Meningitis and pulmonary infections have been treated effectively. Studies in septicemias and urinary tract infections are limited, but good responses have been reported.
Flucytosine Capsules should be used in combination with amphotericin B for the treatment of systemic candidiasis and cryptococcosis because of the emergence of resistance to Flucytosine Capsules (see MICROBIOLOGY).
CONTRAINDICATIONS
Flucytosine Capsules should not be used in patients with a known hypersensitivity to the drug.
WARNINGS
Flucytosine Capsules must be given with extreme caution to patients with impaired renal function. Since Flucytosine Capsules are excreted primarily by the kidneys, renal impairment may lead to accumulation of the drug. Flucytosine Capsule serum concentrations should be monitored to determine the adequacy of renal excretion in such patients. Dosage adjustments should be made in patients with renal insufficiency to prevent progressive accumulation of active drug.
Flucytosine Capsules must be given with extreme caution to patients with bone marrow depression. Patients may be more prone to depression of bone marrow function if they: 1) have a hematologic disease, 2) are being treated with radiation or drugs which depress bone marrow, or 3) have a history of treatment with such drugs or radiation. Bone marrow toxicity can be irreversible and may lead to death in immunosuppressed patients. Frequent monitoring of hepatic function and of the hematopoietic system is indicated during therapy.
PRECAUTIONS
General
Before therapy with Flucytosine Capsules is instituted, electrolytes (because of hypokalemia) and the hematologic and renal status of the patient should be determined (see WARNINGS). Close monitoring of the patient during therapy is essential.
Laboratory Tests
Since renal impairment can cause progressive accumulation of the drug, blood concentrations and kidney function should be monitored during therapy. Hematologic status (leucocyte and thrombocyte count) and liver function (alkaline phosphatase, SGOT and SGPT) should be determined at frequent intervals during treatment as indicated.
Drug Interactions
Cytosine arabinoside, a cytostatic agent, has been reported to inactivate the antifungal activity of Flucytosine Capsules by competitive inhibition. Drugs which impair glomerular filtration may prolong the biological half-life of flucytosine.
Drug/Laboratory Test Interactions
Measurement of serum creatinine levels should be determined by the Jaffé reaction, since Flucytosine Capsules do not interfere with the determination of creatinine values by this method. Most automated equipment for measurement of creatinine makes use of the Jaffé reaction.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Flucytosine has not undergone adequate animal testing to evaluate carcinogenic potential. The mutagenic potential of flucytosine was evaluated in Ames-type studies with five different mutants of S. typhimurium and no mutagenicity was detected in the presence or absence of activating enzymes. Flucytosine was nonmutagenic in three different repair assay systems (i.e., rec, uvr and pol).
There have been no adequate trials in animals on the effects of flucytosine on fertility or reproductive performance. The fertility and reproductive performance of the offspring (F1 generation) of mice treated with 100 mg/kg/day (345 mg/M2/day or 0.059 times the human dose), 200 mg/kg/day (690 mg/M2/day or 0.118 times the human dose) or 400 mg/kg/day (1380 mg/M2/day or 0.236 times the human dose) of flucytosine on days 7 to 13 of gestation was studied; the in utero treatment had no adverse effect on the fertility or reproductive performance of the offspring.
Pregnancy
Teratogenic Effects
Flucytosine was shown to be teratogenic (vertebral fusions) in the rat at doses of 40 mg/kg/day (298 mg/M2/day or 0.051 times the human dose) administered on days 7 to 13 of gestation. At higher doses (700 mg/kg/day; 5208 mg/M2/day or 0.89 times the human dose administered on days 9 to 12 of gestation), cleft lip and palate and micrognathia were reported. Flucytosine was not teratogenic in rabbits up to a dose of 100 mg/kg/day (1423 mg/M2/day or 0.243 times the human dose) administered on days 6 to 18 of gestation. In mice, 400 mg/kg/day of flucytosine (1380 mg/M2/day or 0.236 times the human dose) administered on days 7 to 13 of gestation was associated with a low incidence of cleft palate that was not statistically significant. Studies in pregnant rats have shown that flucytosine injected intraperitoneally crosses the placental barrier. There are no adequate and well-controlled studies in pregnant women. Flucytosine Capsules should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Flucytosine Capsules, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The efficacy and safety of Flucytosine Capsules have not been systematically studied in pediatric patients. A small number of neonates have been treated with 25 to 200 mg/kg/day of flucytosine, with and without the addition of amphotericin B, for systemic candidiasis. No unexpected adverse reactions were reported in these patients. It should be noted, however, that hypokalemia and acidemia were reported in one patient who received flucytosine in combination with amphotericin B, and anemia was observed in a second patient who received flucytosine alone. Transient thrombocytopenia was noted in two additional patients, one of whom also received amphotericin B.
ADVERSE REACTIONS
The adverse reactions which have occurred during treatment with Flucytosine Capsules are grouped according to organ system affected.
Cardiovascular: Cardiac arrest, myocardial toxicity, ventricular dysfunction.
Respiratory: Respiratory arrest, chest pain, dyspnea.
Dermatologic: Rash, pruritus, urticaria, photosensitivity.
Gastrointestinal: Nausea, emesis, abdominal pain, diarrhea, anorexia, dry mouth, duodenal ulcer, gastrointestinal hemorrhage, acute hepatic injury including hepatic necrosis with possible fatal outcome in debilitated patients, hepatic dysfunction, jaundice, ulcerative colitis, enterocolitis, bilirubin elevation, increased hepatic enzymes.
Genitourinary: Azotemia, creatinine and BUN elevation, crystalluria, renal failure.
Hematologic: Anemia, agranulocytosis, aplastic anemia, eosinophilia, leukopenia, pancytopenia, thrombocytopenia, and fatal cases of bone marrow aplasia.
Neurologic: Ataxia, hearing loss, headache, paresthesia, parkinsonism, peripheral neuropathy, pyrexia, vertigo, sedation, convulsions.
Psychiatric: Confusion, hallucinations, psychosis.
Miscellaneous: Fatigue, hypoglycemia, hypokalemia, weakness, allergic reactions, Lyell's syndrome.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
There is no experience with intentional overdosage. It is reasonable to expect that overdosage may produce pronounced manifestations of the known clinical adverse reactions. Prolonged serum concentrations in excess of 100 mcg/mL may be associated with an increased incidence of toxicity, especially gastrointestinal (diarrhea, nausea, vomiting), hematologic (leukopenia, thrombocytopenia) and hepatic (hepatitis).
In the management of overdosage, prompt gastric lavage or the use of an emetic is recommended. Adequate fluid intake should be maintained, by the intravenous route if necessary, since Flucytosine Capsules are excreted unchanged via the renal tract. The hematologic parameters should be monitored frequently; liver and kidney function should be carefully monitored. Should any abnormalities appear in any of these parameters, appropriate therapeutic measures should be instituted.
Since hemodialysis has been shown to rapidly reduce serum concentrations in anuric patients, this method may be considered in the management of overdosage.
DOSAGE AND ADMINISTRATION
The usual dosage of Flucytosine Capsules is 50 to 150 mg/kg/day administered in divided doses at 6-hour intervals. Nausea or vomiting may be reduced or avoided if the capsules are given a few at a time over a 15-minute period. If the BUN or the serum creatinine is elevated, or if there are other signs of renal impairment, the initial dose should be at the lower level (see WARNINGS).
Flucytosine Capsules should be used in combination with amphotericin B for the treatment of systemic candidiasis and cryptococcosis because of the emergence of resistance to Flucytosine Capsules (see MICROBIOLOGY).
HOW SUPPLIED
Flucytosine Capsules are supplied as capsules containing 500 mg flucytosine.
500 mg Capsules (gray and white), imprinted ANCOBON® 500 ICN, available in bottles of 100 (NDC: 63629-2148-1).
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
| FLUCYTOSINE
flucytosine capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Registrant - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(63629-2148) , RELABEL(63629-2148) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.