MAGNESIUM HYDROXIDE/ALUMINUM HYDROXICE/SIMETHICONE- magnesium hydroxide,aluminum hydroxice,simethicone suspension MAGNESIUM HYDROXIDE/ALUMINUM HYDROXICE/SIMETHICONE suspension
Magnesium Hydroxide/Aluminum Hydroxice/Simethicone by
Drug Labeling and Warnings
Magnesium Hydroxide/Aluminum Hydroxice/Simethicone by is a Otc medication manufactured, distributed, or labeled by Major Pharmaceuticals, Plastikon Healthcare, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
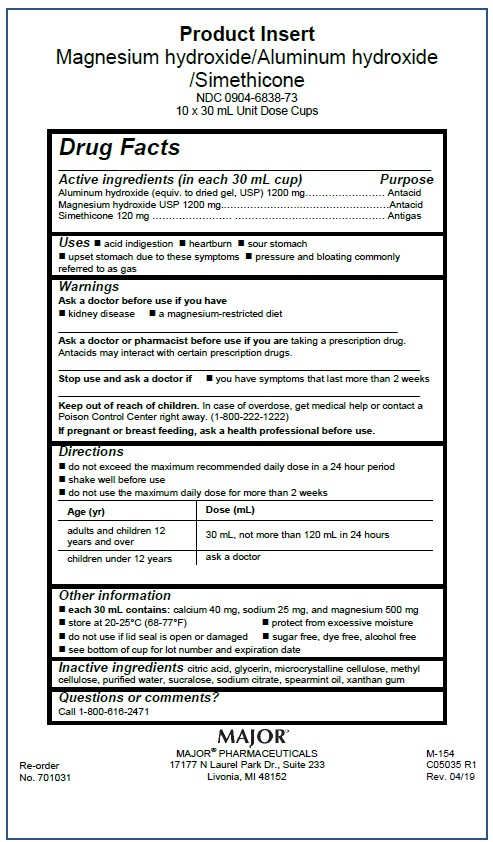
Magnesium hydroxide, Aluminum hydroxide, Simethicone 1200/1200/120 mg per 30 mL
Major Pharmaceutical OTC Monograph
 NDC: 0904-6838-73
NDC: 0904-6838-73
Each 30 mL Contains:
Magnesium Hydroxide1200 mg
Aluminum Hydroxide 1200 mg
Simethicone 120 mg
Delivers 30 mL
Shake Well
See Insert
For Instituional Use Only
MAJOR PHARMACEUTICALS
Livonia, MI 64152
Sugar Free - Dye Free - Alcohol Free
MgOH, AlOH, Simethicone 1200/1200/120 mg/30 mL
Directions
- do not exceed the maximum recommended daily dose in a 24 hour period
- shake well before use
- do not use the maximum daily dose for more than 2 weeks
Age (yr)
Dose (mL)
adults and children 12 years and over
30 mL, not more than 120 mL in 24 hours
children under 12 years
ask a doctor
MgOH, AlOH, Simethicone 1200/1200/120 mg/30 mL
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
MgOH, AlOH, Simethicone 1200/1200/120 mg/30 mL
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium-restricted diet
___________________________________________________________________
Ask a doctor or pharmacist before use if you are taking a presecription drug. Antacids may interact with certain prescription drugs.
___________________________________________________________________
Stop use and ask a doctor if
- you have symptoms that last more than 2 weeks
___________________________________________________________________
If pregnant or breast-feeding, ask a health professional before use.
MgOH, AlOH, Simethicone 1200/1200/120 mg/30 mL
Inactive ingredients citric acid, glycerin, microcrystalline cellulose, methyl cellulose, purified water, saccharin sodium, sodium citrate, spearmint oil, xantham gum
MgOH, AlOH, Simethicone 1200/1200/120 mg/30 mL
Uses
- acid indigestion
- heartburn
- sour stomach
- upset stomach due to these symptoms
- pressure and bloating commonly referred to as gas
MgOH, AlOH, Simethicone 1200/1200/120 mg/30 mL
Active ingredient (in each 30 mL cup)
Magnesium hydroxide USP 1200 mg
Magnesium hydroxide USP 1200 mg
Simethicone 120 mg
MgOH, AlOH, Simethicone 1200/1200/120 mg/30 mL
Other information
- each 30 mL contains: calcium 40 mg, sodium 25 mg, and magnesium 500 mg
- store at 20-25°C (68-77°F)
- protect from excessive moisture
- do not use if lid seal is open or damaged
- sugar free, dye free, alcohol free
- see bottom of cup for lot number and expiration date
-
Magnesium hydroxide, Aluminum hydroxide, Simethicone 2400/2400/240 mg/30 mL
Major Pharmaceutical OTC Monograph
 NDC: 0904-6839-73
NDC: 0904-6839-73
Each 30 mL Contains:
Magnesium Hydroxide 2400 mg
Aluminum Hydroxide 2400 mg
Simethicone 240 mg
Max
Delivers 30 mL
Shake Well
See Insert
For Instituional Use Only
MAJOR PHARMACEUTICALS
Livonia, MI 64152
Sugar Free - Dye Free - Alcohol Free
Magnesium hydroxide, Aluminum hydroxide, Simethicone 2400/2400/240 mg/30 mL
Directions
- do not exceed the maximum recommended daily dose in a 24 hour period
- shake well before use
- do not use the maximum daily dose for more than 2 weeks
Age (yr)
Dose (mL)
adults and children 12 years and over
30 mL, not more than 60 mL in 24 hrs.
children under 12 years
ask a doctor
Magnesium hydroxide, Aluminum hydroxide, Simethicone 2400/2400/240 mg/30 mL
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Magnesium hydroxide, Aluminum hydroxide, Simethicone 2400/2400/240 mg/30 mL
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium-restricted diet
Ask a doctor or pharmacist before use if you are taking a prescription drug. Antacids may interact with certain prescription drugs.
___________________________________________________________________
Stop use and ask a doctor if
- you have symptoms that last for more than 2 weeks.
___________________________________________________________________
If pregnant or breast-feeding, ask a health professional before use.
Magnesium hydroxide, Aluminum hydroxide, Simethicone 2400/2400/240 mg/30 mL
Inactive ingredients citric acid, glycerin, microcrystalline cellulose, methyl cellulose, purified water, saccharin sodium, sodium citrate, spearmint oil, xanthan gum
Magnesium hydroxide, Aluminum hydroxide, Simethicone 2400/2400/240 mg/30 mL
Uses
- acid indigestion
- heartburn
- sour stomach
- upset stomach due to these symptoms
- pressure and bloating commonly referred to as gas
Magnesium hydroxide, Aluminum hydroxide, Simethicone 2400/2400/240 mg/30 mL
Other information
- each 30 mL contains: calcium 40 mg, sodium 25 mg, and magnesium 1000 mg
- store at 20-25°C (68-77°F) -
- protect from excessive moisture
- do not use if lid seal is open or damaged -
- sugar free, dye free, alcohol free
- see bottom of cup for lot number and expiration date
-
INGREDIENTS AND APPEARANCE
MAGNESIUM HYDROXIDE/ALUMINUM HYDROXICE/SIMETHICONE
magnesium hydroxide,aluminum hydroxice,simethicone suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-6839 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 240 mg in 30 mL ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 2400 mg in 30 mL MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (HYDROXIDE ION - UNII:9159UV381P, MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM HYDROXIDE 2400 mg in 30 mL Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) XANTHAN GUM (UNII: TTV12P4NEE) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) METHYLCELLULOSE (15 CPS) (UNII: NPU9M2E6L8) SUCRALOSE (UNII: 96K6UQ3ZD4) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Product Characteristics Color white (Suspension) Score Shape Size Flavor SPEARMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-6839-73 10 in 1 CASE 12/27/2019 1 10 in 1 TRAY 1 30 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 12/27/2019 MAGNESIUM HYDROXIDE/ALUMINUM HYDROXICE/SIMETHICONE
magnesium hydroxide/aluminum hydroxice/simethicone suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-6838 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 120 mg in 30 mL ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 1200 mg in 30 mL MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (HYDROXIDE ION - UNII:9159UV381P, MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM HYDROXIDE 1200 mg in 30 mL Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) XANTHAN GUM (UNII: TTV12P4NEE) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) METHYLCELLULOSE (15 CPS) (UNII: NPU9M2E6L8) SUCRALOSE (UNII: 96K6UQ3ZD4) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Product Characteristics Color white (Suspension) Score Shape Size Flavor SPEARMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-6838-73 10 in 1 CASE 12/27/2019 1 10 in 1 TRAY 1 30 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 12/27/2019 Labeler - Major Pharmaceuticals (191427277) Registrant - Plastikon Healthcare, LLC (041717941) Establishment Name Address ID/FEI Business Operations Plastikon Healthcare, LLC 041717941 manufacture(0904-6838, 0904-6839)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.