OMISIRGE- omidubicel-onlv kit
OMISIRGE by
Drug Labeling and Warnings
OMISIRGE by is a Other medication manufactured, distributed, or labeled by Gamida Cell Inc., Gamida Cell LTD, Hy Laboratories Ltd., Alcami Carolinas Corporation, Eurofins Biolab S.r.l, Aminolab Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OMISIRGE safely and effectively. See full prescribing information for OMISIRGE.
OMISIRGE® (omidubicel-onlv) Suspension for Intravenous Use
Initial U.S. Approval: 2023WARNING: GRAFT VERSUS HOST DISEASE, INFUSION REACTIONS, AUTOIMMUNE CYTOPENIAS, GRAFT FAILURE, and ENGRAFTMENT SYNDROME
See full prescribing information for complete boxed warning.
- Graft-vs-Host Disease (GvHD): GvHD may be fatal. Administration of immunosuppressive therapy may decrease the risk of GvHD. (5.1)
- Infusion reactions: Infusion reactions may be fatal. Monitor patients during infusion and discontinue for severe reactions. Use is contraindicated in patients with known allergy to dimethyl sulfoxide (DMSO), Dextran 40, gentamicin, human serum albumin, or bovine material. (4, 5.2)
- Autoimmune cytopenias: Autoimmune cytopenias have occurred following treatment of severe aplastic anemia. Monitor blood counts prior to and after infusion. Manage cytopenias according to local institutional guidelines. (5.3)
- Graft failure: Graft failure may be fatal. Monitor patients for laboratory evidence of hematopoietic recovery. (5.4)
- Engraftment syndrome: Engraftment syndrome may be fatal. Treat engraftment syndrome promptly with corticosteroids. (5.6)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
OMISIRGE is a nicotinamide modified allogeneic hematopoietic progenitor cell therapy derived from cord blood indicated for the treatment of:
- adults and pediatric patients 12 years and older with hematologic malignancies who are planned for umbilical cord blood transplantation following myeloablative conditioning to reduce the time to neutrophil recovery and the incidence of infections. (1.1)
- adults and pediatric patients 6 years and older with severe aplastic anemia (SAA) following reduced intensity conditioning. (1.2)
DOSAGE AND ADMINISTRATION
For intravenous use only.
Do not irradiate.
- Do not use a leukodepleting filter. (2)
- Verify patient's identity upon receipt, prior to thaw and prior to infusion. (2)
- Thawing should only take place immediately prior to use. (2)
- Premedicate the patient approximately 30 to 60 minutes prior to infusion. (2)
- The recommended dose of OMISIRGE is a one-time infusion delivered in two separate bags. (2)
- The CF (Cultured Fraction) bag must be administered FIRST, and infusion should not exceed 2 hours from the end of dilution. Infusion of the NF (Non-cultured) bag should not exceed 1 hour from the end of dilution. (2)
- Administration of OMISIRGE should be under the supervision of a physician experienced in treatment of hematologic malignancies or SAA, as appropriate, in centers with expertise in hematopoietic stem cell transplants. (2)
- See full prescribing information for details for preparation and administration of OMISIRGE.
DOSAGE FORMS AND STRENGTHS
A single dose of OMISIRGE consists of
- a Cultured Fraction (CF): a minimum of 8.0 × 108 total viable cells of which a minimum of 8.7% is CD34+ cells and a minimum of 9.2 × 107 CD34+ cells, and
- a Non-cultured Fraction (NF): a minimum of 4.0 × 108 total viable cells with a minimum of 2.4 × 107 CD3+ cells. (3)
CONTRAINDICATIONS
Known sensitivity to dimethyl sulfoxide (DMSO), Dextran 40, gentamicin, human serum albumin or bovine material. (4)
WARNINGS AND PRECAUTIONS
- Malignancies of donor origin: Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs after treatment with OMISIRGE, contact Gamida Cell at (844) 477-7478. (5.5)
- Transmission of serious infections: Monitor patients closely for serious infections. (5.7)
- Transmission of rare genetic diseases: Monitor patients for rare genetic diseases. (5.8)
ADVERSE REACTIONS
- Hematological malignancies: The most common adverse reactions (incidence > 20%) are infections, GvHD, and infusion and hypersensitivity reactions. (6.1)
- SAA: The most common adverse reactions (incidence > 20%) are infections, hyperglycemia, skin rash, febrile neutropenia, immune thrombocytopenia, acute kidney injury, acute GvHD, hypertension, hypoxia, and infusion related reactions. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Gamida Cell at (844) 477-7478 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2026
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: GRAFT VERSUS HOST DISEASE, INFUSION REACTIONS, AUTOIMMUNE CYTOPENIAS, GRAFT FAILURE, and ENGRAFTMENT SYNDROME
1 INDICATIONS AND USAGE
1.1 Hematologic Malignancies
1.2 Severe Aplastic Anemia
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Graft-versus-Host Disease
5.2 Hypersensitivity and Infusion-Related Reactions
5.3 Autoimmune Cytopenias
5.4 Graft Failure
5.5 Malignancies of Donor Origin
5.6 Engraftment Syndrome
5.7 Transmission of Serious Infections
5.8 Transmission of Rare Genetic Diseases
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2. Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Hematologic Malignancies
14.2 Severe Aplastic Anemia
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: GRAFT VERSUS HOST DISEASE, INFUSION REACTIONS, AUTOIMMUNE CYTOPENIAS, GRAFT FAILURE, and ENGRAFTMENT SYNDROME
- Graft-vs-Host Disease (GvHD): GvHD may be fatal. Administration of immunosuppressive therapy may decrease the risk of GvHD [see Warnings and Precautions (5.1)].
- Infusion reactions: Infusion reactions may be fatal. Monitor patients during infusion and discontinue for severe reactions. Use is contraindicated in patients with known allergy to dimethyl sulfoxide (DMSO), Dextran 40, gentamicin, human serum albumin, or bovine material [see Contraindications (4), Warnings and Precautions (5.2)].
- Autoimmune cytopenias: Autoimmune cytopenias have occurred following treatment of severe aplastic anemia. Monitor blood counts prior to and after infusion. Manage cytopenias according to local institutional guidelines [see Warnings and Precautions (5.3)].
- Graft failure: Graft failure may be fatal. Monitor patients for laboratory evidence of hematopoietic recovery [see Warnings and Precautions (5.4)].
- Engraftment syndrome: Engraftment syndrome may be fatal. Treat engraftment syndrome promptly with corticosteroids [see Warnings and Precautions (5.6)].
-
1 INDICATIONS AND USAGE
1.1 Hematologic Malignancies
OMISIRGE is indicated for the treatment of adults and pediatric patients 12 years and older with hematologic malignancies who are planned for umbilical cord blood transplantation following myeloablative conditioning to reduce the time to neutrophil recovery and the incidence of infections.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dose
For intravenous use only.
The recommended dose of OMISIRGE is a one-time infusion delivered in two separate bags which consists of
- a Cultured Fraction (CF): a minimum of 8.0 × 108 total viable cells of which a minimum of 8.7% is CD34+ cells and a minimum of 9.2 × 107 CD34+ cells, and
- a Non-cultured Fraction (NF): a minimum of 4.0 × 108 total viable cells with a minimum of 2.4 × 107 CD3+ cells
The CF and NF are supplied cryopreserved. OMISIRGE requires thaw and dilution with two infusion solution (IS) bags (one IS bag for the CF, and one IS bag for the NF) prior to administration. Infusion of the NF bag should begin within 1 hour after completion of the CF infusion. For timing of dosing of each fraction, refer to section 2.2 under "Planning prior to OMISIRGE preparation".
2.2 Preparation and Administration
Administration of OMISIRGE should be under the supervision of a physician experienced in treatment of hematologic malignancies or SAA, as appropriate, in centers with expertise in hematopoietic stem cell transplants.
Preparation
Pretreatment
- Confirm the Release For Infusion Certificate (RFI Certificate) is available for OMISIRGE before starting the conditioning regimen.
-
Before infusion of OMISIRGE, administer as appropriate:
- For patients with hematologic malignancies, administer a myeloablative conditioning regimen according to institutional guidelines.
- For patients with SAA, administer a reduced intensity conditioning regimen according to institutional guidelines.
-
Administer prophylactic and supportive therapies [including Granulocyte-Colony Stimulating Factor (G-CSF)] for prevention or treatment of transplant complications (GvHD, infections) according to institutional guidelines.
Confirm emergency medications are available prior to infusion and during the recovery period as per institutional guidelines.
Premedication for Patients with Hematologic Malignancies
- Premedicate the patient approximately 30 to 60 minutes prior to OMISIRGE infusion.
- Premedicate with diphenhydramine 50 mg IV (or 0.5 mg/kg up to a maximum of 50 mg) or dexchlorpheniramine 10 mg IV, hydrocortisone 50 mg IV (or 0.5 mg/kg up to a maximum of 50 mg) and acetaminophen 650 mg PO (or 10 mg/kg up to a maximum of 650 mg).
- Avoid prophylactic use of methylprednisolone in conjunction with OMISIRGE.
- Ensure the patient is adequately hydrated.
Premedication for Patients with SAA
- For patients receiving Anti-thymocyte globulin (ATG) – administer oral prednisone at 1 mg/kg/day (or IV methylprednisolone if clinically indicated) 1 day prior to the first dose of ATG and continue according to institutional guidelines.
- Administer diphenhydramine 25-50 mg PO or IV and acetaminophen 650 mg PO or weight-based dosing for pediatric patients as per institutional guidelines, approximately 30 minutes prior to OMISIRGE infusion.
- Ensure the patient is adequately hydrated.
Receipt of OMISIRGE
Do not irradiate.
OMISIRGE is shipped directly to the transplant center in 2 shipping containers: [see How supplied/ Storage and Handling (16)]
- A liquid nitrogen dry vapor shipper containing the CF, the NF and a Chimerism Testing Sample(s) at ≤ - 150℃.
- A refrigerated shipping container containing the Infusion Solution for CF and the Infusion Solution for NF at 2-8℃.
- Confirm that the batch number and patient-specific identifiers on both shipping container labels match the intended patient and the information on the documents from the Gamida Cell Assist Hospital Portal.
- Confirm receipt of the Release for Shipping Certificate. Confirm patient-specific identifiers on the RFI Certificate and Certificates of Analysis (CoAs) match the patient's identity.
- Ensure that OMISIRGE was received in appropriate conditions and confirm that the temperature of the liquid nitrogen dry vapor shipper upon receipt was ≤ -150℃ and the temperature of the refrigerated shipping container was 2-8℃.
- If either of the shippers have expired upon arrival, or if you cannot confirm the patient identity with the patient-specific identifiers on any of the labels, contact Gamida Cell at (844) 477-7478.
- You should receive a total of 4 bags [i.e., CF Drug Product (DP) bag, NF DP bag, IS bag for CF DP and IS bag for NF DP] and vial or segment(s) containing Chimerism Testing Sample(s) in the OMISIRGE shipment.
The liquid nitrogen dry vapor shipper contains two metal cassettes, one labeled for the CF containing the CF cryopreserved bag and one labeled for the NF containing the NF cryopreserved bag. The shipper also contains a Chimerism Testing Sample(s).
- Verify that the products are within their expiry date by checking the label located on the front of the metal cassettes on the cryopreserved bags.
- Verify that the patient-specific identifiers on the labels on the outside of the CF and NF metal cassettes and on the CF and NF cryopreserved bags (see Figure 1) match the intended patient.
- Transfer the metal cassettes containing the CF and NF cryopreserved bags and the Chimerism Testing Sample(s) to onsite vapor phase of liquid nitrogen storage at ≤ -150℃.
Figure 1: CF or NF Cryopreserved Bag inside closed Metal Cassette.
Patient-specific identifiers are visible on the cryopreserved bag and can be seen through the cassette window.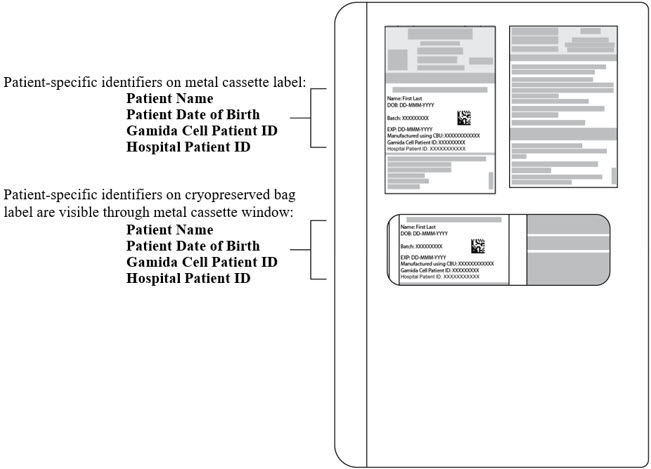
The refrigerated shipping container contains 2 IS bags, the IS for CF and the IS for NF, each with tubing and an attached spike adaptor. Each IS bag is packed inside a sterile bag.
- Ensure that both bags are intact and verify that the Infusion Solutions are within their expiry date by checking the expiration date on the labels located on the IS bags.
- Verify that the patient-specific identifiers on the IS bag labels match the intended patient (see Figure 2).
- Transfer both IS bags to refrigerated storage at 2-8°C.
Figure 2: Infusion Solution for CF Bag with Patient-Specific Label.
The IS bag has tubing with an attached spike adaptor and is packed inside a sterile bag.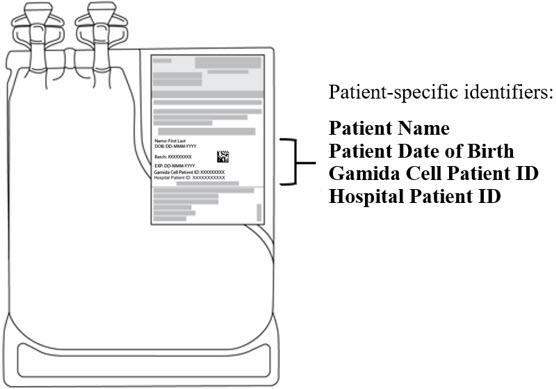
Planning prior to OMISIRGE preparation
- OMISIRGE must not be prepared until after receipt of the RFI Certificate for this patient-specific batch of OMISIRGE. CoAs for the CF, NF and IS batches are attached to the RFI Certificate. The RFI certificate will be issued via Gamida Cell Assist Hospital Portal up to 72 hours after completion of manufacturing.
- Confirm receipt of the RFI Certificate. Confirm patient-specific identifiers on the RFI Certificate and CoAs match the patient's identity.
- The CF bag must be administered FIRST.
- Confirm the infusion time in advance and adjust the start time of CF cryopreserved bag thaw so that it will be available for infusion when the patient is ready.
- Once the CF cryopreserved bag is removed from the metal cassette, thawing and dilution must be carried to completion and the cells administered within 2 hours post-dilution.
- Do not thaw the NF cryopreserved bag until you have determined that the CF has been safely administered.
- Once the NF cryopreserved bag is removed from the metal cassette, thawing and dilution must be carried to completion and the cells administered within 1 hour post-dilution.
- The infusion of the NF bag should begin within 1 hour after completion of the CF infusion.
Preparation of OMISIRGE for Infusion
- Follow universal precautions and local biosafety guidelines for handling and disposal of human cells to avoid potential transmission of infectious diseases.
- Use aseptic technique for all processing steps, including spiking of all transfusion infusion bag ports. No samples should be drawn from OMISIRGE.
The Cultured Fraction
Preparation of the Infusion Solution for CF
- 1. Remove the IS for CF bag from the 2-8°C storage location. Remove only the IS for CF bag at this time.
- 2. Confirm patient-specific identifiers on the label of the IS for CF match the intended patient.
- 3. Wipe the IS for CF sterile bag with 70% alcohol. Place it in the Biological Safety Cabinet (BSC) (if available), for at least 20 minutes with a maximum of 24 hours at room temperature.
- 4. Prior to dilution, remove the IS for CF bag from its sterile bag. Check that the pinch clamp is closed.
Thawing and diluting the CF
- 5. Remove the CF metal cassette from the liquid nitrogen storage.
- 6. Prior to preparing the bag for infusion verify that the patient-specific identifiers on the label on the outside of the cassette and on the CF cryopreserved bag match the intended patient (see Figure 1).
- 7.
Once patient identity has been verified, remove the CF cryopreserved bag from the cassette. Leave the CF cryopreserved bag in the overwrap bag during thawing and dilution.
- 8. Visually inspect the CF cryopreserved bag for damage. If the bag is damaged, contact Gamida Cell at (844) 477-7478. The cryopreserved CF should be white in color.
- 9. Once the CF cryopreserved bag is removed from the metal cassette, the thaw and dilution must be carried to completion and the cells administered within 2 hours post-dilution.
- 10. Incubate the CF cryopreserved bag for 5 minutes at room temperature.
- 11. Place the CF cryopreserved bag in an approximately 37°C water bath until the product reaches a liquid consistency. This generally takes about 3-8 minutes.
Do not massage, knead or apply pressure on the product bag. Keep the bag fully submerged until thawed – do not remove before thawing completion.
- 12. Remove the thawed bag from the water bath as soon as the cells have completely thawed. Do not remove the overwrap bag.
- 13. Wipe the overwrap with 70% alcohol. Put the bag into the BSC (if available).
- 14.
Open the overwrap as follows:
- – Wipe a pair of clean scissors with 70% alcohol.
- –
Cut the sealed area at the top of the overwrap.
Be careful not to damage the CF bag or the CF bag's ports/ tubing.
- 15. Insert the spike adapter attached to the IS for CF bag into one of the ports of the CF bag, while it remains in the overwrap bag.
- 16. Open the pinch clamp on the IS tubing and double the volume of the CF by adding IS for CF (approximately 20 mL) to the CF bag. Gently swirl the bag until mixed well.
- 17. Add the remaining IS for CF (approximately 60 mL) to the CF bag. Close the valve and swirl gently.
- 18. Remove the overwrap of the CF bag and check the integrity of the CF bag.
- 19. Check the appearance of the contents of the CF bag. The thawed and diluted CF should appear as a yellowish suspension, essentially free of visible white clumps and foreign particulates.
- 20. Inspect the contents of the thawed and diluted CF bag for any visible cell clumps. If visible cell clumps remain, gently invert and/or massage the bag with fingertips. Small clumps of cellular material should disperse with gentle manual mixing. Do not infuse the CF if clumps are not dispersed, the bag is damaged or leaking, or otherwise appears to be compromised. If this occurs, call Gamida Cell at (844) 477-7478.
- 21. Heat seal and detach the emptied Infusion Solution for CF bag.
- 22. Connect the Transfusion Infusion Set to the free port on the CF bag. Alternatively, the infusion set may be connected in accordance with internal procedures.
- 23. Place the CF bag containing the thawed and diluted CF in a new sterile bag.
Note: Do not wash, spin down, and/or resuspend CF in new media prior to infusion.
- 24.
Transport the product to the patient at room temperature. Unless prepared at the patient's bedside, transport the product to the bedside in a closed box/bag to protect the product during transport.
The CF bag should be completely infused within 2 hours post-dilution. - 25. See the ‘Administration’ section on how to infuse the CF.
The Non-cultured Fraction
Preparation of the Infusion Solution for NF
- 1. Remove the IS for NF bag from the 2-8°C storage location.
- 2. Repeat steps 2-4 from the CF process, for the IS for NF.
Thawing and diluting the NF
- 3. Repeat steps 5-8 from the CF process, for the NF. The cryopreserved NF should be red in color.
- 4. Once the NF cryopreserved bag is removed from the metal cassette, the thaw and dilution must be carried to completion and the cells administered within 1 hour post-dilution.
- 5. Repeat steps 10-14 from the CF process, for the NF.
- 6. Insert the spike adapter attached to the IS for NF bag into one of the ports of the NF bag, while it remains in the overwrap bag.
- 7. Open the pinch clamp on the IS tubing and double the volume of the NF by adding Infusion Solution for NF (approximately 10 mL) to the NF bag. Gently swirl the bag until mixed well.
- 8. Add the remaining IS for NF (approximately 30 mL) to the NF bag. Close the valve and swirl gently.
- 9. Repeat steps 18-21 from the CF process, for the NF. The thawed and diluted NF should appear as a reddish suspension essentially free of visible clumps and foreign particulates.
- 10. Repeat steps 22-25 from the CF process, for the NF. The NF should be completely infused within 1 hour post-dilution.
Administration
Do NOT use a leukodepleting filter
- Central venous access is recommended for the infusion of OMISIRGE.
- Confirm that the patient's identity matches the patient-specific identifiers on the CF and NF bags.
- Administer OMISIRGE by gravity infusion.
- Prior to spiking both the CF and NF bags, prime the infusion set tubing with normal saline.
- Infuse the entire contents of the CF and NF bags.
- The rate of infusion should not exceed a maximum of 10 mL per kg per hour.
Administration:
- The thawed and diluted CF bag must be infused FIRST. The infusion time should not exceed 2 hours from the end of dilution to the end of CF infusion. Should an infusion reaction occur, appropriately manage the reaction before thawing the NF.
- The thawed and diluted NF should be infused within 1 hour of safely administering the CF infusion. The infusion time should not exceed 1 hour from the end of dilution to the end of infusion.
- In the event of any deviation from the dosing schedule, contact Gamida Cell at (844) 477-7478.
- After the entire contents of the CF and NF bags are each infused, wash the tubing with normal saline at the same infusion rate to ensure as many cells as possible are delivered to the patient.
Follow universal precautions and local biosafety guidelines for handling and disposal of human cells to avoid potential transmission of infectious diseases.
Monitoring
- Monitor the patient for hypersensitivity or other infusion-related reactions during the infusion and post-infusion, per institutional guidelines.
- Reduce the infusion rate if the fluid load is not tolerated. Pause the infusion in the event of a hypersensitivity reaction or if the patient develops a moderate to severe infusion reaction. Administer appropriate medical therapy as needed. [See Warnings and Precautions (5.2)]
- Conduct frequent clinical and laboratory assessments and vital signs and monitor for graft failure, GvHD, infections and other post-transplant complications according to institutional guidelines.
-
3 DOSAGE FORMS AND STRENGTHS
A single dose of OMISIRGE consists of:
- a Cultured Fraction: At the time of cryopreservation, the CF contains a minimum of 8.0 × 108 total viable cells with a minimum of 8.7% CD34+ cells and a minimum of 9.2 × 107 CD34+ cells suspended in approximately 10% dimethyl sulfoxide (DMSO).
- a Non-cultured Fraction: At the time of cryopreservation, the NF contains a minimum of 4.0 × 108 total viable cells with a minimum of 2.4 × 107 CD3+ cells suspended in approximately 10% DMSO.
Each fraction is supplied separately in its own cryopreserved bag [see How Supplied/Storage and Handling (16)]. Both bags diluted with their respective IS must be infused to achieve the dose of OMISIRGE.
See the respective CoA for the CF and NF for actual cell counts. The CoAs are attached to the RFI Certificate available via the Gamida Cell Assist Hospital Portal.
Table 1: Appearance of OMISIRGE Product Bags Appearance Immediately before Thawing Appearance Post-Dilution Cultured Fraction White, frozen at the bottom of the cryopreserved bag Yellowish suspension, essentially free of visible white clumps and foreign particulates Non-cultured Fraction Red, frozen at the bottom of the cryopreserved bag Reddish suspension, essentially free of visible clumps and foreign particulates - 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Graft-versus-Host Disease
Acute and chronic graft-versus-host disease (GvHD) have occurred following treatment with OMISIRGE [see Adverse Reactions (6.1)]. Acute GvHD manifests as maculopapular rash, gastrointestinal symptoms, and elevated bilirubin. Chronic GvHD manifests as skin rash, oral symptoms, ocular dryness, transaminase elevations, gastrointestinal symptoms, or serositis.
Patients treated with OMISIRGE should receive immunosuppressive drugs to decrease the risk of GvHD, and be monitored for signs and symptoms of GvHD, and treated if GvHD develops.
5.2 Hypersensitivity and Infusion-Related Reactions
Hypersensitivity and infusion-related reactions have occurred with OMISIRGE administration [see Adverse Reactions (6.1)].
Serious hypersensitivity reactions, including anaphylaxis, may be due to DMSO, residual gentamicin, Dextran 40, human serum albumin (HSA) and bovine material in OMISIRGE. OMISIRGE may contain residual antibiotics if the cord blood donor was exposed to antibiotics in utero. Patients with a history of allergic reactions to antibiotics should be monitored for allergic reactions following OMISIRGE administration.
Signs and symptoms of hypersensitivity reactions may include bronchospasm, wheezing, angioedema, pruritus, hives, fever, and hypotension during or after OMISIRGE infusion.
Infusion-related reactions may begin within minutes of the start of infusion of OMISIRGE, although symptoms may continue to intensify and not peak for several hours after the completion of the infusion.
Premedicate patients with antipyretics, histamine antagonists, and corticosteroids and monitor closely for signs and symptoms of hypersensitivity and infusion-related reactions. When a reaction occurs, pause the infusion and institute supportive care as needed.
5.3 Autoimmune Cytopenias
Autoimmune cytopenias (AICs) have occurred with OMISIRGE administration in patients with SAA. AIC is characterized by thrombocytopenia, anemia, and neutropenia, alone or in combination, occurring weeks to months post-transplant, often after initial hematopoietic recovery.
Risk factors for post-transplant AIC include younger age, ATG-containing conditioning, underlying SAA, and delayed T cell chimerism.
Monitor blood counts prior to and after OMISIRGE infusion. Manage cytopenias according to local institutional guidelines.
5.4 Graft Failure
Graft failure has occurred with OMISIRGE administration [see Adverse Reactions (6.1)]. Primary graft failure, which may be fatal, is defined as failure to achieve an absolute neutrophil count greater than 500 per microliter blood by Day 42 after transplantation. Immunologic rejection is the primary cause of graft failure. Patients should be monitored for laboratory evidence of hematopoietic recovery.
5.5 Malignancies of Donor Origin
Malignancy of donor origin including post-transplant lymphoproliferative disorder (PTLD) has occurred with OMISIRGE administration. PTLD manifests as a lymphoma-like disease favoring non-nodal sites. PTLD is usually fatal if not treated. The etiology is thought to be donor lymphoid cells transformed by Epstein-Barr virus (EBV). Serial monitoring of blood for EBV DNA may be warranted in patients with persistent cytopenias.
A donor-cell derived myelodysplastic syndrome (MDS) has occurred with OMISIRGE administration. The natural history is presumed to be the same as that for de novo MDS. Monitor life-long for secondary malignancies.
In the event that a secondary malignancy occurs, contact Gamida Cell at (844) 477-7478.
5.6 Engraftment Syndrome
Engraftment syndrome may occur because OMISIRGE is derived from umbilical cord blood. Monitor patients for unexplained fever, rash, hypoxemia, weight gain, and pulmonary infiltrates in the peri-engraftment period. Treat with corticosteroids as soon as engraftment syndrome is recognized to ameliorate symptoms. If untreated, engraftment syndrome may progress to multiorgan failure and death.
5.7 Transmission of Serious Infections
Transmission of infectious disease may occur because OMISIRGE is derived from umbilical cord blood. Disease may be caused by known or unknown infectious agents. Donors are screened for increased risk of infection with human immunodeficiency virus (HIV), human T-cell lymphotropic virus (HTLV), hepatitis B virus (HBV), hepatitis C virus (HCV), T. pallidum, West Nile virus (WNV), transmissible spongiform encephalopathy (TSE) agents, vaccinia, and Zika virus [for umbilical cord blood (UCB) collected between March 2016 and 20 May 2024]. Donors are also screened for clinical evidence of sepsis, and communicable disease risks associated with xenotransplantation. Maternal blood samples are tested for HIV types 1 and 2, HTLV types I and II, HBV, HCV, T. pallidum, and WNV. OMISIRGE is tested for sterility. There may be an effect on the reliability of the sterility test results if the cord blood donor was exposed to antibiotics in utero. OMISIRGE is tested for sterility, endotoxin, and mycoplasma. These measures do not totally eliminate the risk of transmitting these or other transmissible infectious diseases and disease agents.
Testing of maternal and infant donor blood is also performed for evidence of donor infection due to cytomegalovirus (CMV).
Test results may be found on the container label and/or in accompanying records.
Product manufacturing includes bovine-derived reagents. While all animal-derived reagents are tested for animal viruses, bacteria, fungi, and mycoplasma before use, these measures do not eliminate the risk of transmitting these or other transmissible infectious diseases and disease agents.
Final sterility test results may not be available at the time of use, but Quality Assurance (QA) will communicate any positive results from sterility testing to the physician. Report the occurrence of transmitted infection to Gamida Cell at (844) 477-7478.
5.8 Transmission of Rare Genetic Diseases
OMISIRGE may transmit rare genetic diseases involving the hematopoietic system because it is derived from umbilical cord blood. Cord blood donors have been screened to exclude donors with sickle cell anemia, and anemias due to abnormalities in hemoglobins C, D, and E. Because of the age of the donor at the time cord blood collection takes place, the ability to exclude rare genetic diseases is severely limited. Report the occurrence of transmitted rare genetic disease to Gamida Cell at (844) 477-7478.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Hematologic Malignancies
The safety of OMISIRGE is based on data from Study P0501 for 52 patients transplanted with OMISIRGE and 56 patients transplanted with umbilical cord blood (UCB) [see Clinical Studies (14)]. The median duration of follow up for the overall safety population was 14 months (range, 1-19 months). All patients received myeloablative preparative regimens and GvHD prophylaxis with tacrolimus or cyclosporin plus mycophenolate mofetil.
Fatal adverse reactions occurred in 17% of patients treated with OMISIRGE, including infection (6%), acute GvHD (6%), veno-occlusive disease (VOD)/sinusoidal obstruction syndrome (SOS) (2%), thrombotic thrombocytopenic purpura (TTP)/thrombotic microangiopathy (TMA) (2%), and pulmonary hemorrhage (2%). Fatal adverse reactions occurred in 29% of subjects treated with UCB, including infection/sepsis (11%), respiratory disorders (11%), GvHD (5%), and VOD/SOS (2%).
The most common non-laboratory adverse reactions occurring in ≥ 10% of patients in Study P0501 are listed in Table 2 below. The most common Grade 3-5 adverse reactions for patients treated with OMISIRGE, were pain (33%), mucosal inflammation (31%), hypertension (25%), and gastrointestinal toxicity (19%).
Table 2: Adverse Reactions in ≥ 10% of Patients with Hematologic Malignancies Following Transplantation with OMISIRGE (N=52) or UCB (N=56) in Study P0501 Adverse Reaction OMISIRGE
Any Grade
n (%)OMISIRGE
Grade 3 or Higher n (%)UCB
Any Grade
n (%)UCB
Grade 3 or Higher n (%)Abbreviation: n: number; UCB: umbilical cord blood. - * Fatigue includes asthenia and fatigue.
- † Infections and infestations were graded according to the BMT-CTN grading system.
- ‡ Acute Graft-versus-host disease was graded according to the Consensus Conference on Acute GvHD grading.
- § Chronic Graft-versus-host disease was graded according to the 2014 NIH consensus criteria.
- ¶ Hemorrhage include cystitis hemorrhagic, epistaxis, gastrointestinal hemorrhage, hemorrhage, pulmonary alveolar hemorrhage, subarachnoid hemorrhage, and upper gastrointestinal hemorrhage.
- # Respiratory failure includes acute respiratory distress syndrome, acute respiratory failure, hypoxia, and respiratory failure.
- Þ Renal impairment includes acute kidney injury, blood creatinine increased and renal failure.
General disorders and administration site conditions - - - - Pain 41(79) 17 (33) 43 (77) 10 (18) Fever 42 (81) 1 (2) 54 (96) 6 (11) Mucosal inflammation 39 (75) 16 (31) 47 (84) 19 (34) Fatigue* 31(60) 2 (4) 42 (75) 12 (21) Edema 24 (46) 1 (2) 37 (66) 4 (7) Chills 19 (37) 0 32 (57) 0 Gastrointestinal disorders - - - - Gastrointestinal toxicity 40 (77) 10 (19) 48 (86) 19 (34) Vomiting 33 (63) 3 (6) 40 (71) 2 (4) Dysphagia 17 (33) 6 (12) 21 (38) 7 (13) Constipation 12 (23) 0 21 (38) 0 Dyspepsia 12 (23) 0 12 (21) 0 Abdominal distention 10 (19) 0 16 (29) 1 (2) Infections and infestations† - - - - Viral infections 39 (75) 4 (8) 45 (80) 15 (27) Bacterial infections 34 (65) 4 (8) 45 (80) 13 (23) Fungal infections 11 (21) 3 (6) 15 (27) 10 (18) Immune System Disorder - - - - Acute Graft versus host disease‡ 32 (62) 8 (15) 24 (43) 12 (21) Chronic Graft versus host disease§ 18 (35) 12 (23) 14 (25) 11 (20) Vascular disorders - - - - Hypertension 29 (56) 13 (25) 37 (66) 21 (38) Hemorrhage¶ 25 (48) 6 (12) 34 (61) 10 (18) Hypotension 16 (31) 2 (4) 19 (34) 5 (9) Psychiatric disorders - - - - Insomnia 24 (46) 1 (2) 26 (46) 2 (4) Anxiety 15 (29) 1 (2) 21(38) 3 (5) Depression 13 (25) 0 16 (29) 2 (4) Cardiac Disorders - - - - Arrythmia 24 (46) 0 30 (54) 1 (2) Investigations - - - - Weight decrease/Decreased appetite 23 (44) 4 (8) 22 (39) 1 (2) Musculoskeletal and connective tissue disorders - - - - Muscular weakness 16 (31) 1 (2) 22 (39) 2 (4) Nervous system disorder - - - - Dysgeusia 15 (29) 0 9 (16) 0 Dizziness 10 (19) 0 13 (23) 0 Tremor 8 (15) 0 12 (21) 1 (2) Somnolence 7 (13) 1 (2) 12 (21) 0 Respiratory, thoracic, and mediastinal disorders - - - - Cough 14 (27) 0 30 (54) 0 Dyspnea 13 (25) 4 (8) 26 (46) 9 (16) Dehydration 11 (21) 3 (6) 10 (18) 2 (4) Respiratory Failure# 8 (15) 6 (12) 26 (46) 17 (30) Neoplasms benign, malignant and unspecified (incl cysts and polyps) - - - - Disease recurrence 11 (21) 8 (15) 7 (13) 5 (14) Renal and urinary disorders - - - - Renal impairmentÞ 9 (17) 6 (12) 3 (5) 3 (5) Eye disorders - - - - Dry eyes 6 (12) 0 10 (18) 0 Injury, poisoning and procedural complications - - - - Primary graft failure 1 (2) 1 (2) 6 (11) 6 (11) Secondary graft failure 1 (2) 1 (2) 0 0 Table 3 summarizes selected chemistry abnormalities by treatment arm for patients treated in Study P0501.
Table 3: Chemistry Laboratory Abnormalities in ≥10% of Patients in Study P0501 OMISIRGE
N = 52UCB
N = 56Laboratory Abnormality Grade 1-4
%Grade 3-4
%Grade 1-4
%Grade 3-4
%Abbreviation: N: number; UCB: umbilical cord blood. Decreased magnesium 94 4 91 2 Increased aspartate aminotransferase 56 13 61 7 Increased alanine aminotransferase 56 13 57 9 Increased creatinine 50 4 57 2 Increased bilirubin 42 12 61 21 Increased alkaline phosphatase 42 0 54 2 Increased magnesium 15 2 29 9 Severe Aplastic Anemia
The safety data described in this section reflects exposure of OMISIRGE in one clinical study (Study 17-H-0091) for the treatment of severe aplastic anemia (SAA). A total of 17 patients received a single dose of OMISIRGE with a median dose of 8.5 × 106 cells/kg CD34+ cells (range, 2.3- 21.4 cells/kg CD34+ cells). Three out of 17 patients received OMISIRGE with haploidentical CD34+ cells [see Clinical Studies (14)]. All patients received a reduced intensity preparative conditioning regimen of cyclophosphamide, fludarabine, TBI and horse-ATG, and GvHD prophylaxis according to institutional guidelines. The median duration of follow-up was 25 months (range, 2-60 months).
Serious adverse reactions were reported in 15 patients including infections (n=15), diarrhea (n=3), nausea/vomiting (n=4), pyrexia (n=2), hypoxia (n=2), thrombotic microangiopathy (n=1), cardiac arrest (n=1), pericarditis (n=1), colitis (n=1), febrile neutropenia (n=1), cholecystitis (n=1), portal vein thrombosis (n=1), graft-versus-host disease (n=1), weight decreased (n=1), dehydration (n=1), Guillain-Barre Syndrome (n=1), uterine hemorrhage (n=1), pleural effusion (n=1), pulmonary hemorrhage (n=1), and respiratory failure (n=1).
One patient (6%) treated with OMISIRGE had a fatal adverse event. The patient engrafted but died on Day 62 from disseminated adenovirus infection.
Common Terminology Criteria for Adverse Events (CTCAE) Grade 3-5 non-laboratory adverse reactions in the SAA Study with greater or equal to 15% incidence are summarized in Table 4. The most common Grade 3-5 adverse reactions for patients treated with OMISIRGE were febrile neutropenia (41%), bacterial infections (41%), hyperglycemia (41%), Epstein-Barr virus infection (29%), immune thrombocytopenia (24%) and pneumonia (24%).
Table 4: Adverse Reactions in ≥15% of Patients with SAA Following Transplantation with OMISIRGE (N=17) in Study 17-H-0091 Adverse Reaction OMISIRGE Any Grade n (%) OMISIRGE Grade 3 or Higher n (%) Abbreviation: n: number. - * Includes skin infection, Clostridium difficile infection, device related infections and urinary tract infections.
- † Is a composite that includes multiple related terms.
- ‡ Includes hypertension, headache and hypoxia.
- § Includes dyspnea, respiratory distress and respiratory failure.
Infections and infestations - - Human herpesvirus 6 infection 16 (94) 0 BK virus infection 13 (76) 0 Bacterial infections* 10 (59) 7 (41) Epstein-Barr virus infection 9 (53) 5 (29) Cytomegalovirus infection† 8 (47) 3 (18) Pneumonia 6 (35) 4 (24) Adenovirus infection 4 (24) 1 (6) Rhinovirus infection 4 (24) 1 (6) Sepsis† 3 (18) 3 (18) Upper respiratory tract infection 3 (18) 1 (6) Metabolism and nutrition disorders - - Hyperglycemia† 11 (65) 7 (41) Hypertriglyceridemia 3 (18) 2 (12) Skin and subcutaneous tissue disorders - - Skin rash† 8 (47) 0 Blood and lymphatic system disorders - - Febrile neutropenia† 7 (41) 7 (41) Immune thrombocytopenia 4 (24) 4 (24) Injury, poisoning and procedural complications - - Infusion related reaction‡ 4 (24) 2 (12) Immune system disorders - - Acute graft-versus-host disease 4 (24) 1 (6) Respiratory, thoracic and mediastinal disorders - - Hypoxia 4 (24) 3 (18) Respiratory failure§ 3 (18) 3 (18) Vascular disorders - - Hypertension 4 (24) 3 (18) Renal and urinary disorders - - Acute kidney injury 4 (24) 2 (12) Gastrointestinal disorders - - Diarrhea 3 (18) 3 (18) Nausea 3 (18) 2 (12) Vomiting 3 (18) 2 (12) Other clinically significant adverse reactions occurring in <15% of patients include the following: Post-transplant lymphoproliferative disorder in two patients (12%), primary graft failure (defined as failure to achieve an absolute neutrophil count ≥500 cells / µl for 3 consecutive measurements on different days) in 1 patient (6%), and engraftment syndrome in 1 patient (6%).
Table 5 summarizes laboratory abnormalities that worsened from baseline in ≥ 15% of patients in Study 17-H-0091.
Table 5: Laboratory Abnormalities that Worsened from Baseline in ≥15% of Patients in Study 17-H-0091 (N=17) Laboratory Abnormality Grade 1-4
N (%)Grade 3-4
N (%)Abbreviation: N: number. Decreased potassium 5 (29) 0 Increased potassium 4 (24) 1 (6) Decreased phosphorous 4 (24) 0 Increased alanine aminotransferase 3 (18) 2 (12) -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with OMISIRGE use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with OMISIRGE to assess whether it can cause fetal harm when administered to a pregnant woman. In Study 17-H-0091, one patient reported two pregnancies, one at 9 months and one at 3.5 years post-transplant. There were no reported birth complications or neonatal concerns.
OMISIRGE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In the United States (U.S.) general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of OMISIRGE in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for OMISIRGE and any potential adverse effects on the breastfed infant from OMISIRGE or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy status of females with reproductive potential should be verified. Sexually active females of reproductive potential should have a pregnancy test prior to starting the conditioning regimen for OMISIRGE.
Contraception
See the prescribing information for the medications used for conditioning for information on the need for effective contraception in patients who receive a conditioning regimen.
There are insufficient exposure data to provide a recommendation concerning duration of contraception following treatment with OMISIRGE.
8.4 Pediatric Use
The safety and efficacy of OMISIRGE have been established in pediatric patients with hematologic malignancy 12 years of age and older and in pediatric patients with severe aplastic anemia (SAA) 6 years of age and older.
The use of OMISIRGE in pediatric patients with hematologic malignancy was supported by evidence from one clinical study (Study P0501) which included 2 pediatric patients age 12 to 16 years. The use of OMISIRGE in pediatric patients with SAA was supported by evidence from one clinical study (Study 17-H-0091) which included 7 pediatric patients 6 to 16 years of age.
-
11 DESCRIPTION
OMISIRGE (omidubicel-onlv) is a cryopreserved nicotinamide modified unrelated allogeneic hematopoietic progenitor cell therapy derived from cord blood consisting of 2 cell fractions; a Cultured Fraction (CF) and a Non-cultured Fraction (NF) which are both derived from the same patient-specific cord blood unit (CBU).
1) The CF is a yellowish suspension consisting of allogeneic, hematopoietic CD34+ progenitor cells.
In addition to the CD34+ hematopoietic progenitor cells (HPCs), the CF consists of other cell populations, including more differentiated myelomonocytic cells, dendritic cells and granulocytes. The CF formulation contains a maximum of 35 mg gentamicin. Following manipulation, the cells are washed, formulated into a suspension, and cryopreserved in a patient specific bag in 10% dimethyl sulfoxide (DMSO). The product is thawed and diluted prior to infusion [see Dosage and Administration (2.2), How Supplied/Storage and Handling (16)]. The CF contains approximately 2.42 mg of DMSO. The diluted CF contains ≤ 2% DMSO.
2) The NF is a reddish suspension consisting of allogeneic, hematopoietic mature myeloid and lymphoid cells that are washed, formulated into a suspension, and cryopreserved in a patient specific bag in 10% DMSO. In addition to the mature myeloid and lymphoid cells, the NF consists of other cell populations, including more lineage committed hematopoietic cells. The product is thawed and diluted prior to infusion [see Dosage and Administration (2.2), How Supplied/Storage and Handling (16)]. The NF contains approximately 1.1 mg of DMSO. The diluted NF contains ≤2% DMSO.
Two Infusion Solution bags are also provided for diluting each fraction after thawing, one specifically for the CF and one specifically for the NF. The Infusion Solutions contain 8% w/v HSA and 6.8% w/v Dextran 40 in 0.9% sodium chloride [see Dosage and Administration (2.2)].
The NF and IS are Released for Shipment (RFS) following full release testing, including sterility test. The CF is RFS at the end of manufacturing, prior to final product testing completion. OMISIRGE is Released for Infusion (RFI) after acceptable results from the CF's Rapid Contamination Test for microbial contamination and quantitative PCR-based mycoplasma test are obtained. The RFI includes all CF DP release testing as detailed in the CF CoA, except the pending Colony Forming Unit (CFU) and final Sterility tests results. RFI certificate is accompanied with the respective IS, NF, and CF CoA's. Receipt of the RFI Certificate for the patient-specific batch of OMISIRGE must be confirmed prior to preparation. RFI Certificate receipt is via the Gamida Cell Assist Hospital Portal, approximately 72 h of the end of manufacturing.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
OMISIRGE is a nicotinamide (NAM) modified allogeneic hematopoietic progenitor cell therapy derived from cord blood used as an allogeneic stem cell donor source. OMISIRGE is manufactured utilizing a proprietary NAM based technology producing enriched HPCs.
NAM technology overcomes the induction of accelerated proliferation, differentiation, cellular stress and signaling pathways that are typically activated when HPCs are removed from their natural environment.
Ex-vivo culturing of cord blood derived HPCs in the presence of NAM leads to preservation of their stemness, homing to the bone marrow (BM) and retained engraftment capacity as demonstrated by rapid neutrophil engraftment and multi lineage immune reconstitution as observed in the clinical trials with OMISIRGE.
12.2. Pharmacodynamics
Transplantation with OMISIRGE resulted in rapid and broad immune reconstitution of dendritic cells, monocytes, Natural Killer (NK), CD4+ T cells and CD8+ T cells as early as one-week post-transplantation, and B cells 28 days post transplantation and all lineages throughout the one-year follow-up period. Robust positive linear correlations between the CD34(+) cell content in the OMISIRGE CF, and the reconstitution of T-cells and NK cells were identified. Additionally, dose-response analyses demonstrated a strong correlation between the total CD34+ cell counts and dose / kg for OMISIRGE with the kinetics of neutrophil recovery. The model demonstrated that days to neutrophil recovery decreased with an increase in OMISIRGE CD34(+) cell dose.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Hematologic Malignancies
OMISIRGE was evaluated in Study P0501 (NCT02730299), an open-label, multicenter, randomized study of OMISIRGE transplantation or UCB transplantation following myeloablative conditioning in patients with hematologic malignancies.
In total, 125 patients with the availability of at least one ≥ 4/6 human leukocyte antigen (HLA)-matched (HLA-A, B and DR loci) cord blood unit were randomized to the study, 62 patients were randomized to receive OMISIRGE and 63 patients were randomized to the UCB group. The minimum specifications of OMISIRGE were a Total Nucleated Viable Cell (TNVC) count of 8.0 × 108 cells and CD34+ cell count of 5.6 × 107. Fifty-two patients were transplanted with OMISIRGE at a median CD34+ cell dose of 9.0 × 106 cells/kg (range 2.1 – 47.6 × 106 cells/kg). Fifty-six patients were transplanted in the UCB arm with one or two cord units (66% received two cord units); among patients in whom the post-thaw cell dose was reported (n = 42), the median CD34+ cell dose was 0.2 × 106 cells/kg (range 0.0 – 0.8 × 106 cells/kg). Multiple conditioning regimens were used, including Total Body Irradiation (TBI)-based or chemotherapy-based options.
Demographic and baseline patient characteristics were similarly distributed among the treatment arms. The overall study population included 72 males (58%) and 53 females (42%) with median age 41 years (range: 13–65). Fifty-eight percent of patients were White, 16% were Black, 14% were Asian and 13% were of other races or unknown. Thirteen percent of patients identified as Hispanic or Latino. Forty-eight percent of patients had Acute Myelogenous Leukemia (AML), 33% had Acute Lymphoblastic Leukemia (ALL), 7% had Myelodysplastic Syndrome (MDS), 5% had Chronic myeloid leukemia (CML), 4% had lymphoma and 3% had other rare leukemias. Baseline disease status (remission vs. overt disease) varied depending on the hematologic malignancy. Disease risk index was high/very high for 34% and moderate for 42%. HCT specific comorbidity index was ≥ 3 in 51% and 1-2 in 28% of patients.
Of the patients randomized to OMISIRGE, 8% of patients (5/62) were not able to receive OMISIRGE due to manufacturing failure.
The efficacy of OMISIRGE was established based on time to neutrophil recovery following transplantation and the incidence of BMT CTN Grade 2/3 bacterial or Grade 3 fungal infections through Day 100 following transplantation. The efficacy outcomes are summarized below.
Table 6: Efficacy Results in Patients Randomized to Receive OMISIRGE or UCB in Study P0501 (ITT Population) Efficacy Outcome OMISIRGE
N=62UCB
N=63Absolute Difference
(95% CI)Abbreviation: CI: Confidence interval; N: number; UCB: umbilical cord blood. - * Time to neutrophil recovery was defined as the time from transplantation to the earliest of 3 consecutive measurements on different days with absolute neutrophil count greater than or equal to 0.5 Gi/L assessed with 42 days of follow-up.
- † Median time to neutrophil recovery was estimated by the Kaplan-Meier estimator.
Median time to neutrophil recovery*,† 12 days
(95% CI: 10-15 days)22 days
(95% CI: 19-25 days)10 days
(95% CI: 6-14 days)Incidence of Grade 2/3 bacterial or Grade 3 fungal infections through 100 days following transplantation 39% 60% 22%
(95% CI: 4%-39%)Eighty-seven percent of patients in the OMISIRGE arm and 83% in the UCB arm achieved neutrophil recovery. The median time to neutrophil recovery was 12 days in the OMISIRGE arm and 22 days in the UCB arm. BMT CTN Grade 2/3 bacterial or Grade 3 fungal infections through Day 100 following transplantation occurred in 39% of patients in the OMISIRGE arm and 60% of patients in the UCB arm.
Per Protocol Population:
Among the patients treated with OMISIRGE (n=52), neutrophil recovery with 42 days of follow-up was achieved in 94% of patients at a median of 10 days (95% CI: 8, 12), compared to 89% of patients treated with UCB (n=56), at a median of 20 days (95% CI: 18, 24). BMT CTN Grade 2/3 bacterial or Grade 3 fungal infections by 100 days were reported in 35% of patients treated with OMISIRGE and 61% of patients treated with UCB, respectively.
14.2 Severe Aplastic Anemia
The efficacy of OMISIRGE in patients with severe aplastic anemia (SAA) was evaluated in study 17- H-0091 (NCT 03173937), an open-label, single center study. The study enrolled patients with SAA who had intolerance or failure to respond to immunosuppressive therapy and availability of at least one ≥ 4/8 human leukocyte antigen (HLA)-matched (HLA-A, B, C and DR loci) cord blood unit. Patients were excluded if there was availability of an HLA identical (12/12) matched related or unrelated donor.
In total, 17 patients were treated with OMISIRGE, among them 14 patients were treated with OMISIRGE alone and three patients were treated with both OMISIRGE and haploidentical CD34+ cells. The minimum specifications of OMISIRGE were a Total Nucleated Viable Cell (TNVC) count of 8.0 × 108 cells and CD34+ cell count of 9.2 × 107. The fourteen patients who were transplanted with OMISIRGE alone were included in the efficacy evaluation. The median CD34+ cell dose of OMISIRGE was 9.5 × 106 cells/kg (range, 2.3 - 21.4 cells/kg). All patients received reduced intensity conditioning which included cyclophosphamide, fludarabine, TBI and horse-ATG. GvHD prophylaxis was administered according to institutional guidelines.
The demographic characteristic of the population included the following: median age was 17 years (range: 6 – 45), 10 patients (59%) were male, 6 patients (35%) were Black, 3 patients (18%) were White, 5 patients (29%) were Asian and 3 patients (18%) were of "other races" or "unknown". Twenty-nine percent of patients identified as Hispanic or Latino. Median absolute neutrophil count at baseline was 0.3 × 109/L (range, 0.0 – 1.0) and median platelet count at baseline was 35 × 109/L (range, 10-89). Hematopoietic stem cell transplantation (HSCT) specific comorbidity index was ≥ 3 in 76% and 0-2 in 24% of patients. Twelve percent of patients received 4/8 HLA match, 59% 5/8 HLA match, 18% 6/8 HLA match, 6% 7/8 HLA match and 6% 8/8 HLA match.
The primary efficacy outcome measure was the incidence of early and sustained neutrophil recovery, defined as ANC ≥500 cells/µl for 3 consecutive measurements on different days by Day 26, maintained at Days 42 and 100 posttransplant. Other secondary efficacy outcomes were neutrophil recovery (days to first of three consecutive ANC≥500 cells/µL), red blood cell (RBC) transfusion independence (days to 30-day transfusion independence), platelet recovery ≥20,000/µL (days to first of 3 consecutive platelet count of 20,000/µL with no preceding transfusion in 7 days) and platelet transfusion independence (days to 30-day platelet transfusion independence).
The efficacy results are summarized in Table 7 below.
Table 7: Efficacy Results from Study 17-H-0091 Efficacy Outcome N=14 Abbreviation: CI: Confidence interval; min: Minimum; max: Maximum; N: number. - * 95% CI was calculated by the Clopper-Pearson method.
- † Time to neutrophil recovery was defined as ANC ≥500 cells/µl for 3 consecutive measurements on different days.
- ‡ Based on patients who achieved the events.
Patients with early and sustained neutrophil recovery at 100 days
n (%)
(95% CI)*12 (86%)
(57%, 98%)Time to neutrophil recovery†
Median days (min - max)‡11 (7 – 20) Time to RBC transfusion independence
No. patients who achieved (%)
Median days (min - max) ‡12 (86%)
58.5 (42 – 446)Time to platelet 20,000/µl recovery
No. patients who achieved within a year (%)
Median days (min – max) ‡12 (86%)
31.5 (20 – 197)Time to platelet transfusion independence
No. patients who achieved (%)
Median days (min - max) ‡11 (79%)
53.0 (43 – 93) -
16 HOW SUPPLIED/STORAGE AND HANDLING
OMISIRGE (NDC: 73441-800-04) is shipped in two shipping containers, a liquid nitrogen dry vapor shipper at ≤ -150℃, containing the two cryopreserved cell fractions (CF NDC: 73441-100-01 and NF NDC: 73441-200-01) and a Chimerism Testing Sample(s) and a refrigerated shipping container at 2-8℃, containing two Infusion Solutions (Infusion Solution for CF NDC: 73441-300-01 and Infusion Solution for NF NDC: 73441-400-01). OMISIRGE is shipped to the transplant center for a specific patient.
The Cryopreserved Cell Fractions
OMISIRGE is comprised of two cryopreserved cell fractions, a Cultured Fraction (CF) and a Non-cultured Fraction (NF) each in a separate cryopreserved bag labeled for the specific patient. Each cryopreserved bag is protected by a corresponding transparent overwrap bag and each cryopreserved bag enclosed in its overwrap bag is individually packed in a metal cassette. Both cryopreserved OMISIRGE cell fractions are shipped together in the vapor phase of liquid nitrogen in a liquid nitrogen dry vapor shipper with the Prescribing Information (PI) and a Chimerism Testing Sample(s).
At the time of cryopreservation, the CF contains a minimum of 8.0 × 108 total viable cells with a minimum of 8.7% CD34+ cells and a minimum of 9.2 × 107 CD34+ cells suspended in 20 mL of a cryopreservation solution containing 10% DMSO.
See the CoA for the CF for actual cell counts. CoAs are attached to the RFI Certificate available via the Gamida Cell Assist Hospital Portal.
Upon cryopreservation, the CF appears white and is frozen at the bottom of the cryopreserved bag.
At the time of cryopreservation, the NF contains a minimum of 4.0 × 108 total viable cells with a minimum of 2.4 × 107 CD3+ cells suspended in 10 mL cryopreservation solution containing 10% DMSO.
See the CoA for the NF for actual cell counts.
Upon cryopreservation, the NF appears red and is frozen at the bottom of the cryopreserved bag.
- Match the identity of the patient with the patient-specific identifiers on the cassettes and cryopreserved bag labels upon receipt.
- Store OMISIRGE frozen in the vapor phase of liquid nitrogen (≤ -150°C) in a temperature-controlled system.
- Thaw immediately prior to use [see Dosage and Administration (2.2)].
- Use closed containers when transporting the bags within the facility.
The Refrigerated Infusion Solutions
The Infusion Solutions (IS) used to dilute OMISIRGE CF and NF are provided in two IS bags labeled for the specific patient and for diluting the specific fraction. The IS for diluting the CF contains approximately 80 mL and the IS for diluting the NF contains approximately 40 mL of IS consisting of 6.8% Dextran 40 and 8% HSA. The Infusion Solutions are shipped in a refrigerated shipping container with the PI.
- Match the identity of the patient with the patient-specific identifiers on the IS bag labels upon receipt.
- Store the IS bags in a 2-8℃ refrigerated storage until the time of thaw of the OMISIRGE CF and NF.
-
17 PATIENT COUNSELING INFORMATION
Discuss the following with the patient receiving OMISIRGE:
The recommended course of therapy for OMISIRGE is a single dose for infusion, which is provided by the manufacturer as 2 separate components (CF and NF). OMISIRGE CF and OMISIRGE NF are infused one after the other once the patient has received an appropriate conditioning regimen.
Each OMISIRGE unit is specific to each patient. Ensure that patients understand the risk of manufacturing failure (8% in Study P0501) of OMISIRGE. In case of a manufacturing failure, a second manufacturing attempt may be considered. In this case, while the patient awaits the product, additional therapy (in addition to the preparative conditioning) may be necessary and may increase the risk of adverse events during the pre-infusion period.
Prior to infusion, advise patients of the following risks:
Graft-versus-Host Disease:
Report immediately any signs and symptoms suggestive of graft vs host disease, including rash, diarrhea or yellowing of the eyes [see Warnings and Precautions (5.1), Adverse Reactions (6.1)].
Hypersensitivity and Infusion-Related Reactions:
Report immediately any signs and symptoms of hypersensitivity reactions including wheezing, swelling, itching, or hives [See Warnings and Precautions (5.2)].
Report immediately any signs and symptoms of infusion reactions including fever, chills, fatigue, tachycardia, hypoxia, severe nausea, severe vomiting, diarrhea, muscle pain, joint pain, low blood pressure, high blood pressure, or dizziness/lightheadedness [see Warnings and Precautions (5.2), Adverse Reactions (6.1)].
Autoimmune Cytopenias
Report immediately any signs and symptoms of Autoimmune Cytopenias including pallor and fatigue, tachycardia (from anemia) and mild to moderate splenomegaly, petechiae, easy bruising or prolonged bleeding from cuts, and recurrent bacterial infections, persistent oral ulcers and gingivitis and frequent fevers [See Warnings and Precautions (5.3)].
Graft Failure:
Advise patients that primary graft failure, which may be fatal, can occur [See Warnings and Precautions (5.4), Adverse Reactions (6.1)].
Malignancies of Donor Origin:
Advise patients of the need to contact Gamida Cell at (844)-477-7478 if they are diagnosed with a secondary malignancy after treatment with OMISIRGE [See Warnings and Precautions (5.5), Adverse Reactions (6.1)].
Engraftment Syndrome:
Report immediately any signs and symptoms suggestive of engraftment syndrome including fever, rash, or unexplained weight gain [See Warnings and Precautions (5.6)].
Transmission of Serious Infections:
Advise patients of the risk of transmission of infectious disease [See Warnings and Precautions (5.7)].
Transmission of Rare Genetic Diseases:
Advise patients of the risk of transmission of rare genetic diseases [See Warnings and Precautions (5.8)].
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 20 mL and 10 mL Shipping Label
NDC: 73441-100-01
NDC: 73441-200-01Gamida Cell Patient ID:
Batch:
Expiry Date:omidubicel-onlv
OMISIRGE®Rx Only
Container 1 of 2Cultured Fraction
Non-cultured FractionSHIP TO:
Transplant Center Name:
Address:
Telephone Number:
Receiver Name(s):Product of Israel
Manufactured For and Distributed By: Gamida Cell, Inc. Naples, FL 34102, U.S.
Telephone Number: (844) 477-7478Ship and Store in Vapor Phase Liquid Nitrogen ≤ -150 °C
DO NOT X-RAY
Exempt Human SpecimenPK000113-9
-
PRINCIPAL DISPLAY PANEL - 80 mL and 40 mL Shipping Label
NDC: 73441-300-01
NDC: 73441-400-01Gamida Cell Patient ID:
Batch:
Expiry Date:omidubicel-onlv
OMISIRGE®Rx Only
Container 2 of 2Infusion Solution for
Cultured Fraction
Infusion Solution for
Non-cultured FractionSHIP TO:
Transplant Center Name:
Address:
Telephone Number:
Receiver Name(s):Product of Israel
Manufactured For and Distributed By: Gamida Cell, Inc. Naples, FL 34102, U.S.
Telephone Number: (844) 477-7478Ship and store at 2–8°C
DO NOT X-RAY
Exempt Human SpecimenPK000112-9

-
INGREDIENTS AND APPEARANCE
OMISIRGE
omidubicel-onlv kitProduct Information Product Type CELLULAR THERAPY Item Code (Source) NDC: 73441-800 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73441-800-04 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BAG 20 mL Part 2 1 BAG 80 mL Part 3 1 BAG 10 mL Part 4 1 BAG 40 mL Part 1 of 4 OMIDUBICEL CULTURED FRACTION
omidubicel cultured fraction suspensionProduct Information Item Code (Source) NDC: 73441-100 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OMIDUBICEL (UNII: ET4JC4S66E) (OMIDUBICEL - UNII:ET4JC4S66E) OMIDUBICEL 800000000 in 100 mL Inactive Ingredients Ingredient Name Strength DIMETHYL SULFOXIDE (UNII: YOW8V9698H) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73441-100-01 20 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125738 05/01/2023 Part 2 of 4 INFUSION SOLUTION FOR CF
infusion solution solutionProduct Information Item Code (Source) NDC: 73441-300 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) DEXTRAN 40 (UNII: K3R6ZDH4DU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73441-300-01 80 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125738 05/01/2023 Part 3 of 4 OMIDUBICEL NON-CULTURED FRACTION
omidubicel non-cultured fraction suspensionProduct Information Item Code (Source) NDC: 73441-200 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OMIDUBICEL NON-CULTURED FRACTION (UNII: MAH7ZHD7ZJ) (OMIDUBICEL NON-CULTURED FRACTION - UNII:MAH7ZHD7ZJ) OMIDUBICEL NON-CULTURED FRACTION 400000000 in 50 mL Inactive Ingredients Ingredient Name Strength DIMETHYL SULFOXIDE (UNII: YOW8V9698H) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73441-200-01 10 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125738 05/01/2023 Part 4 of 4 INFUSION SOLUTION FOR NF
infusion solution solutionProduct Information Item Code (Source) NDC: 73441-400 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) DEXTRAN 40 (UNII: K3R6ZDH4DU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73441-400-01 40 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125738 05/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125738 05/01/2023 Labeler - Gamida Cell Inc. (093828795) Registrant - Gamida Cell LTD (532501574) Establishment Name Address ID/FEI Business Operations Gamida Cell LTD 532501574 API MANUFACTURE(73441-800) , MANUFACTURE(73441-800) , LABEL(73441-800) , PACK(73441-800) , ANALYSIS(73441-800) Establishment Name Address ID/FEI Business Operations Hy Laboratories Ltd. 600013676 ANALYSIS(73441-800) Establishment Name Address ID/FEI Business Operations Alcami Carolinas Corporation 831351445 ANALYSIS(73441-800) Establishment Name Address ID/FEI Business Operations Alcami Carolinas Corporation 832394535 ANALYSIS(73441-800) Establishment Name Address ID/FEI Business Operations Eurofins Biolab S.r.l 429117112 ANALYSIS(73441-800) Establishment Name Address ID/FEI Business Operations Aminolab Ltd 600672703 ANALYSIS(73441-800)
Trademark Results [OMISIRGE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OMISIRGE 88768530 not registered Live/Pending |
Gamida Cell Inc. 2020-01-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.