INSTAREADY FACE PRIMER- avobenzone, homosalate, octisalate, octocrylene liquid
InstaReady Face Primer by
Drug Labeling and Warnings
InstaReady Face Primer by is a Otc medication manufactured, distributed, or labeled by LINA GALE, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- PDP
-
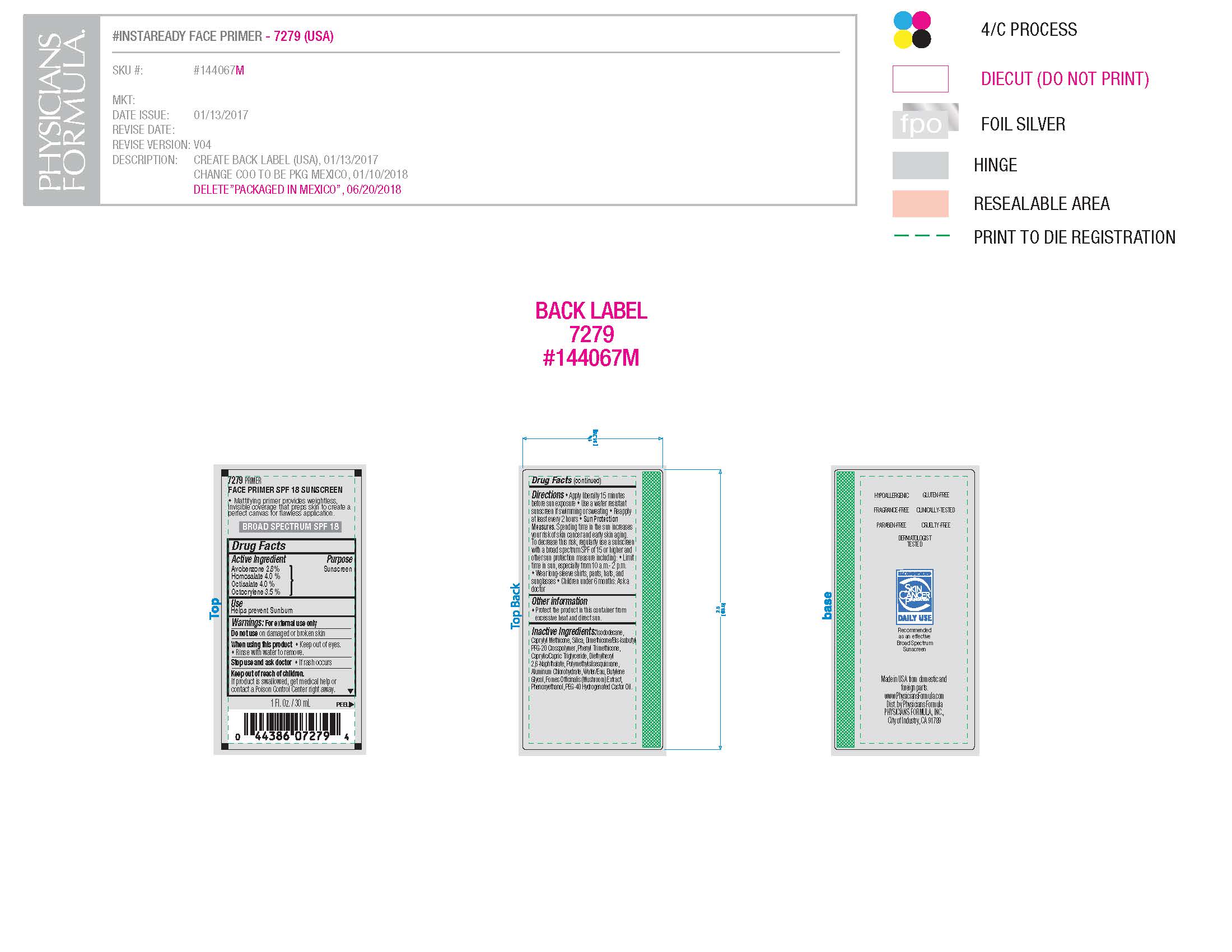
INGREDIENTS AND APPEARANCE
INSTAREADY FACE PRIMER
avobenzone, homosalate, octisalate, octocrylene liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69514-137 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 3.5 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 4 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.8 g in 100 g Inactive Ingredients Ingredient Name Strength DIMETHICONE/BIS-ISOBUTYL PPG-20 CROSSPOLYMER (UNII: O4I3UFO6ZF) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) WATER (UNII: 059QF0KO0R) CAPRYLIC/CAPRIC/PALMITIC/STEARIC TRIGLYCERIDE (UNII: ZF29F7IK5I) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) ISODODECANE (UNII: A8289P68Y2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69514-137-01 30 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/15/2016 Labeler - LINA GALE, INC. (078890119)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.