Sun Face Protector Broad Spectrum 50+
Sun Face Protector Sunscreen Broad Spectrum SPF 50 by
Drug Labeling and Warnings
Sun Face Protector Sunscreen Broad Spectrum SPF 50 by is a Otc medication manufactured, distributed, or labeled by Jafra Cosmetics International Inc, Jafra Manufacturing, S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
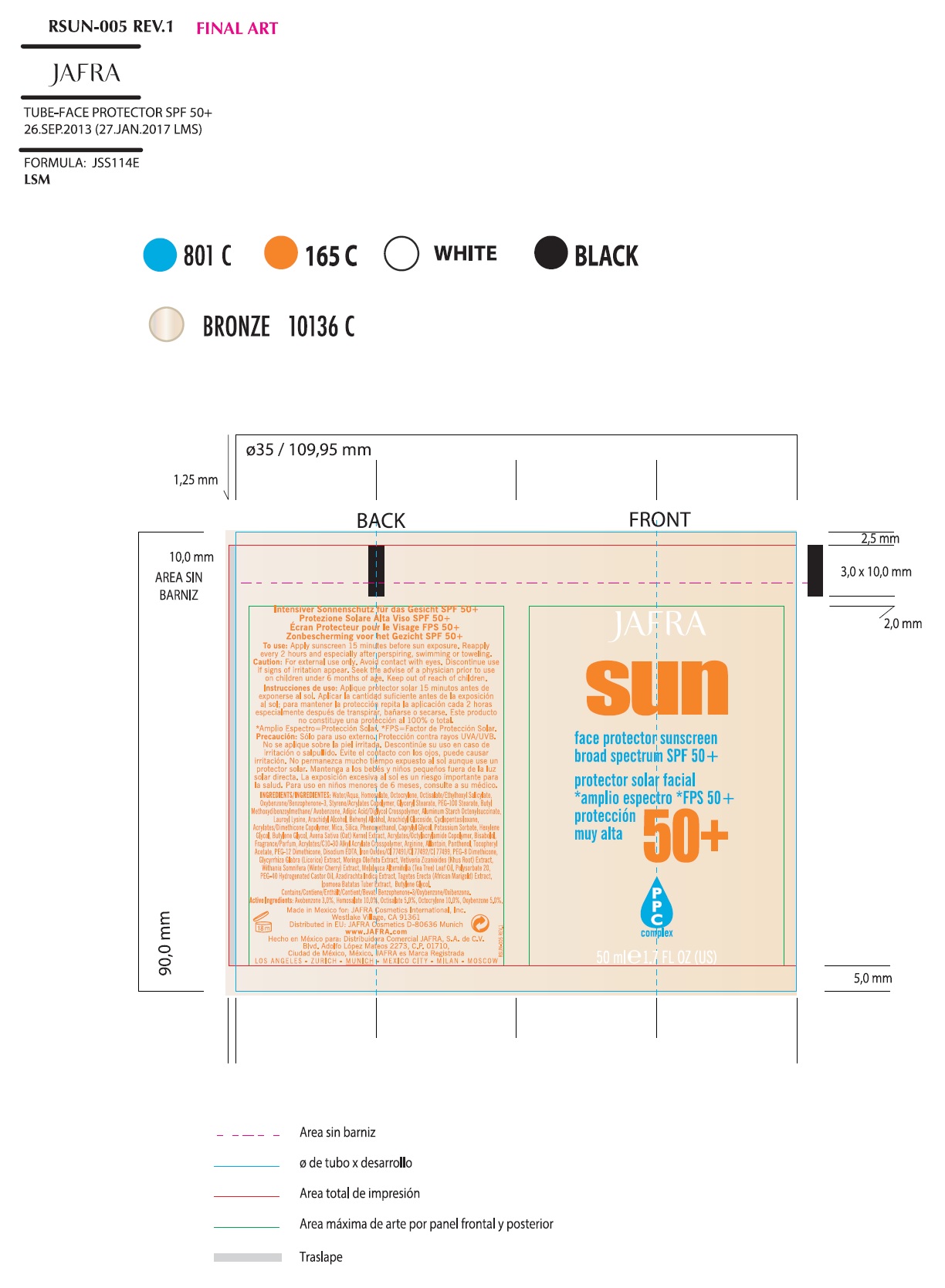
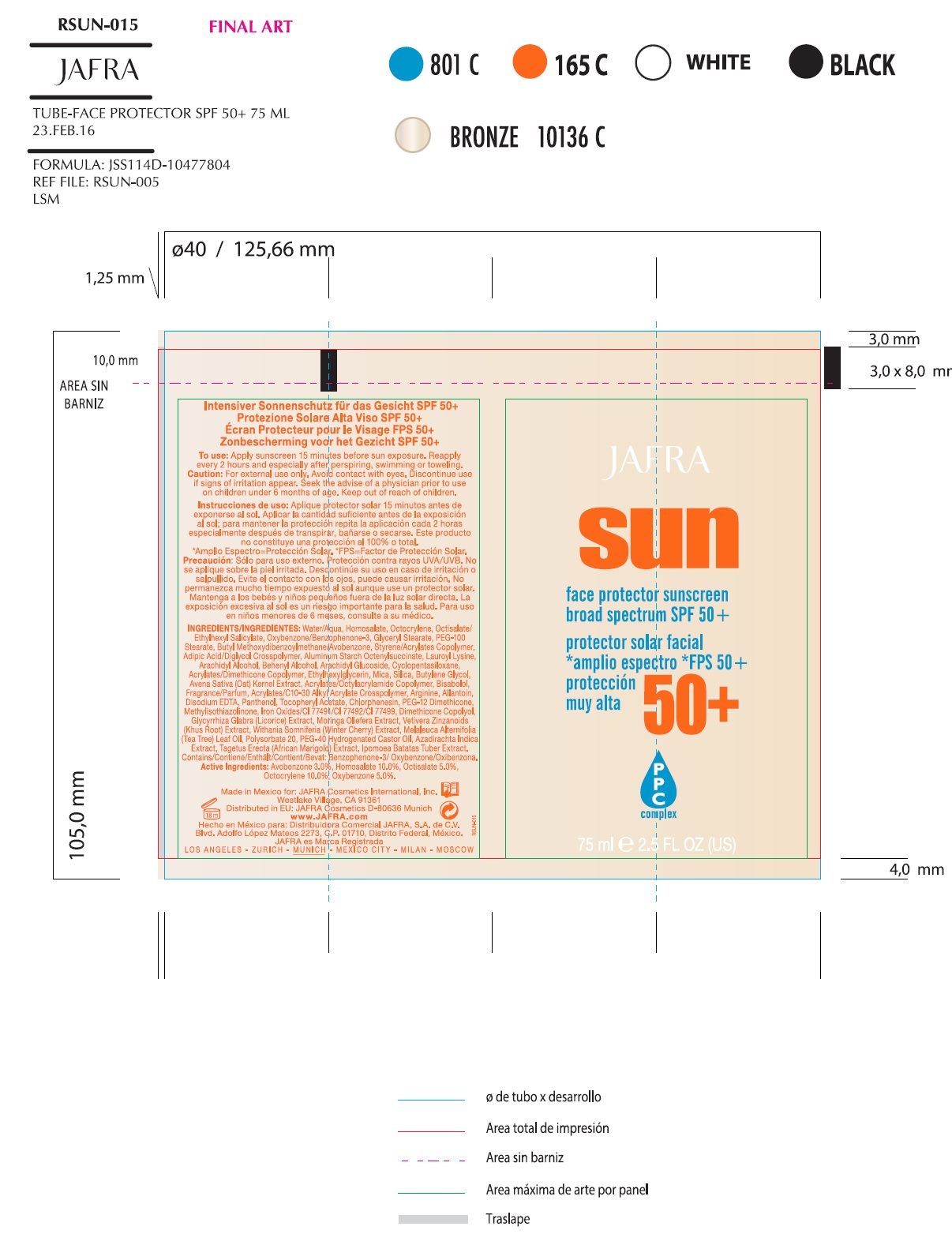
SUN FACE PROTECTOR SUNSCREEN BROAD SPECTRUM SPF 50- avobenzone, homosalate, octisalate,octocrylene, oxybenzone lotion
Jafra Cosmetics International Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Sun Face Protector Broad Spectrum 50+
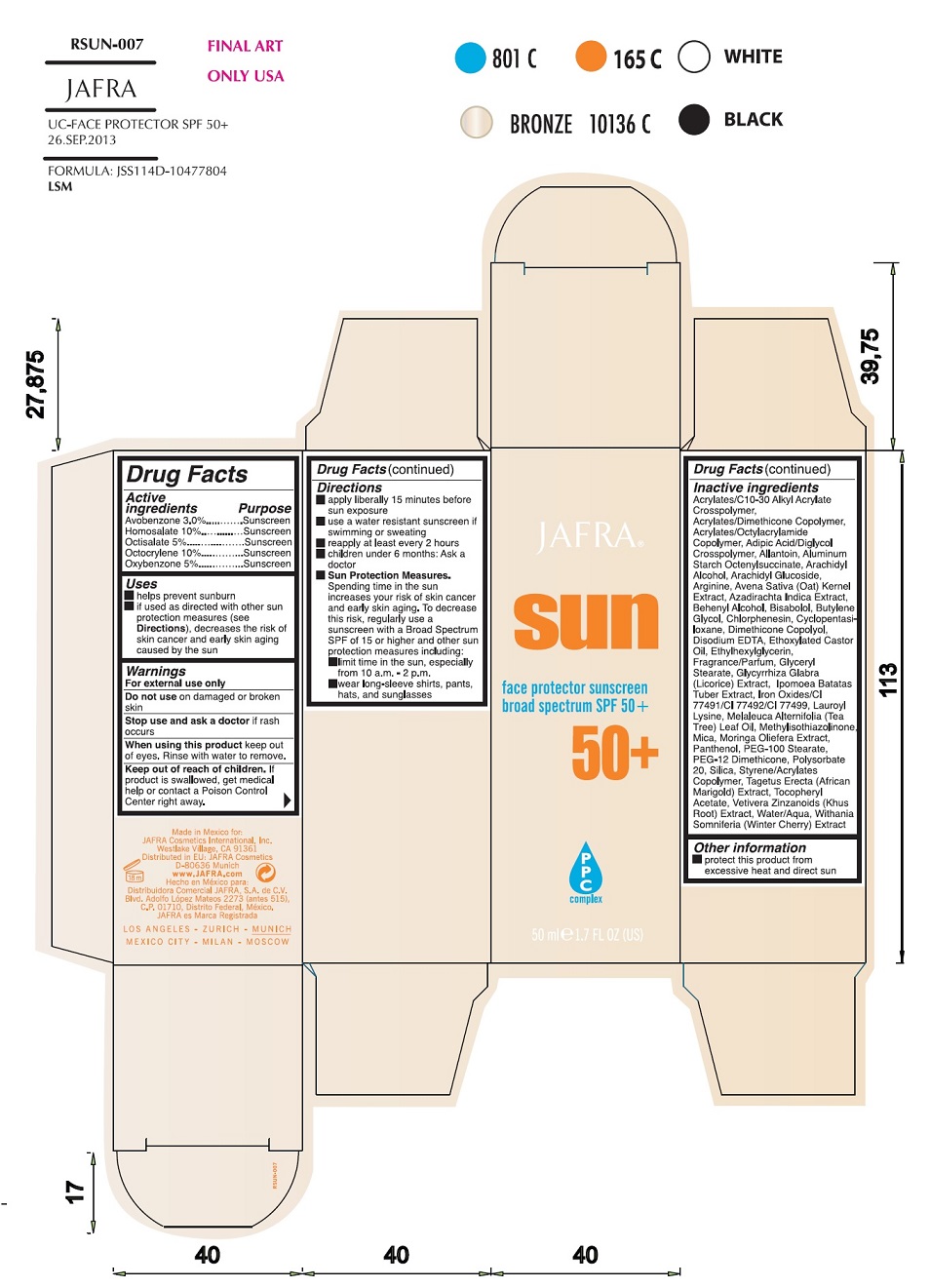
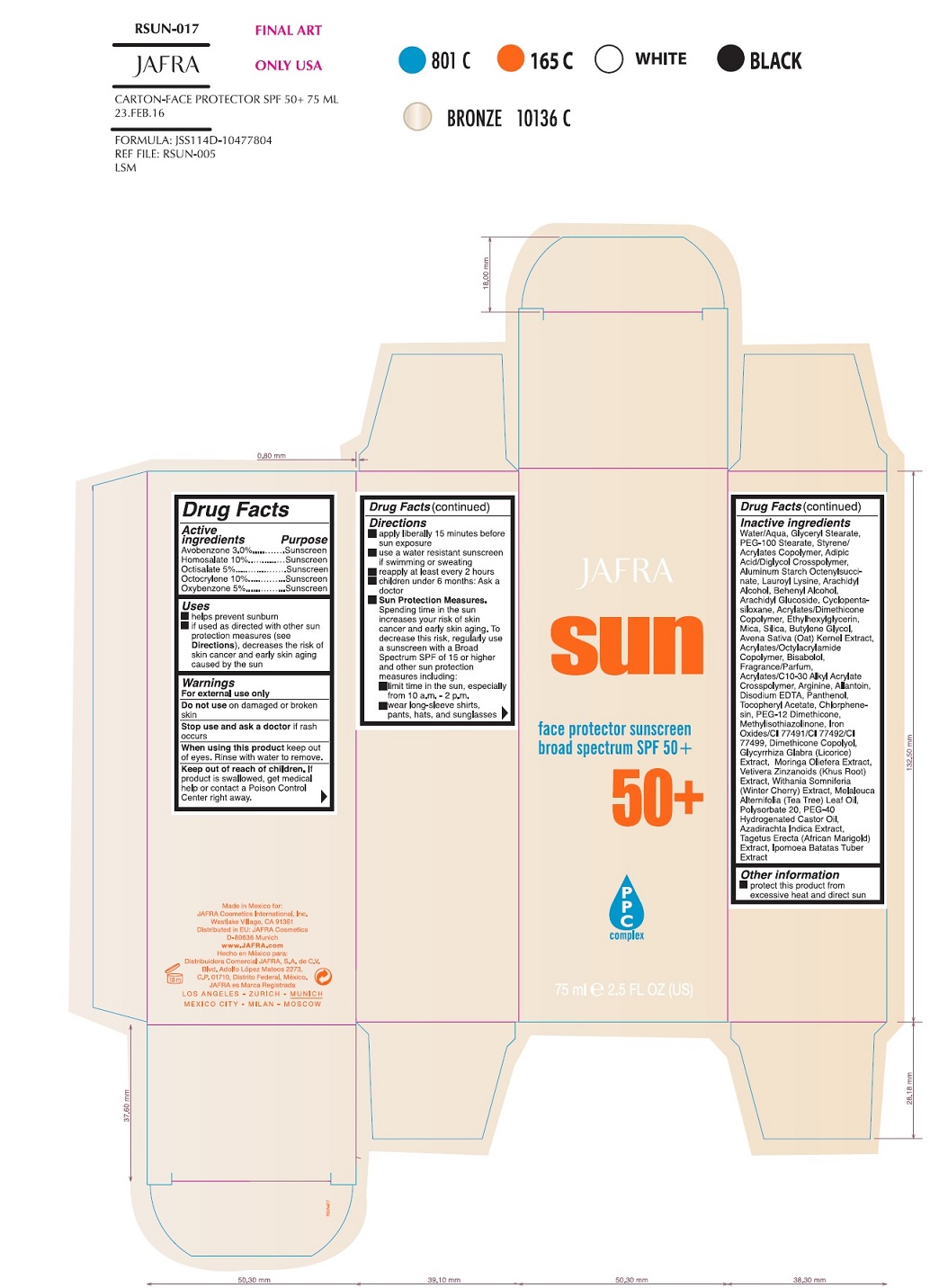
Uses
Helps prevent sunburn
If used as directed with other sun protection measures (see
Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only
Do not useon damaged or broken skin
When using this productkeep out of eyes. Rinse with water to remove.
Stop use and ask a doctorif rash occurs
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center right away.
Directions
apply liberally 15 minutes before sun exposure
use a water resistant sunscreen if swimming or sweating
reapply at least every 2 hours
children under 6 months of age: Ask a doctor
Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
limit your time in the sun, especially from 10 a.m. – 2 p.m.
wear long-sleeved shirts, pants, hats, and sunglasses
Inactive Ingredients
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Acrylates/Dimethicone Copolymer, Acrylates/Octylacrylamide Copolymer, Adipic Acid/Diglycol Crosspolymer, Allantoin, Aluminum Starch Octenylsuccinate, Arachidyl Alcohol, Arachidyl Glucoside, Arginine, Avena Sativa (Oat) Kernel Extract, Azadirachta Indica Extract, Behenyl Alcohol, Bisabolol, Butylene Glycol, Chlorphenesin, Cyclopentasiloxane, Dimethicone Copolyol, Disodium EDTA, Ethoxylated Castor Oil, Ethylhexylglycerin, Fragrance/Parfum, Glyceryl Stearate, Glycyrrhiza Glabra (Licorice) Extract, Ipomoea Batatas Tuber Extract, Iron Oxides/CI 77491/CI 77492/CI 77499, Lauroyl Lysine, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Methylisothiazolinone, Mica, Moringa Oleifera Extract, Panthenol, PEG-100 Stearate, PEG-12 Dimethicone, Polysorbate 20, Silica, Styrene/Acrylates Copolymer, Tagetes Erecta (African Marigold) Extract, Tocopheryl Acetate, Vetiveria Zizanoides (Khus Root) Extract, Water/Aqua, Withania Somnifera (Winter Cherry) Extract
| SUN FACE PROTECTOR SUNSCREEN BROAD SPECTRUM SPF 50
avobenzone, homosalate, octisalate,octocrylene, oxybenzone lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Jafra Cosmetics International Inc (041676479) |
| Registrant - Jafra Cosmetics International Inc (041676479) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Distribuidora Comercial Jafra, S.A. de C.V. | 951612777 | manufacture(68828-752) | |