ACAMPROSATE CALCIUM tablet, delayed release
acamprosate calcium by
Drug Labeling and Warnings
acamprosate calcium by is a Prescription medication manufactured, distributed, or labeled by Zydus Pharmaceuticals (USA) Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use acamprosate calcium delayed-release tablets safely and effectively. See full prescribing information for acamprosate calcium delayed-release tablets.
ACAMPROSATE calcium delayed-release tablets, for oral use
Initial U.S. Approval: 2004INDICATIONS AND USAGE
- Acamprosate calcium delayed-release tablets are indicated for the maintenance of abstinence from alcohol in patients with alcohol dependence who are abstinent at treatment initiation (1, 14).
- Treatment with acamprosate calcium delayed-release tablets should be part of a comprehensive management program that includes psychosocial support (1).
DOSAGE AND ADMINISTRATION
- Recommended dose: 666 mg (two 333 mg tablets) taken three times daily (2).
- Dose reduction to one 333 mg tablet taken three times daily for patients with moderate renal impairment (creatinine clearance 30 to 50 mL/min) (2.1).
- Acamprosate calcium delayed-release tablets are contraindicated in patients with severe renal impairment (creatinine clearance of ≤30 mL/min) (2.1, 4.2, 5.1, 8.6, 12.3).
DOSAGE FORMS AND STRENGTHS
Enteric-coated tablets, 333 mg (3).
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Common adverse events that occurred in any acamprosate calcium treatment group at a rate of 3% or greater and greater than the placebo group in controlled clinical trials with spontaneously reported adverse events are: accidental injury, asthenia, pain, anorexia, diarrhea, flatulence, nausea, anxiety, depression, dizziness, dry mouth, insomnia, paresthesia, pruritus and sweating (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Zydus Pharmaceuticals USA Inc. at 1-877-993-8779 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Pregnancy: Acamprosate calcium should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus (8.1).
- Nursing Mothers: Caution should be exercised when acamprosate calcium is administered to a nursing woman (8.3).
- Renal Impairment: Dose reduction required for moderate renal impairment; contraindicated in severe renal impairment (2.1, 4.2, 5.1, 8.6, 12.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity to Acamprosate Calcium
4.2 Severe Renal Impairment
5 WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
5.2 Suicidality and Depression
5.3 Alcohol Withdrawal
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.3 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Information for Patients
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Acamprosate calcium delayed-release tablets are indicated for the maintenance of abstinence from alcohol in patients with alcohol dependence who are abstinent at treatment initiation. Treatment with acamprosate calcium delayed-release tablets should be part of a comprehensive management program that includes psychosocial support.
The efficacy of acamprosate calcium delayed-release tablets in promoting abstinence has not been demonstrated in subjects who have not undergone detoxification and not achieved alcohol abstinence prior to beginning acamprosate calcium delayed-release tablets treatment. The efficacy of acamprosate calcium delayed-release tablets in promoting abstinence from alcohol in polysubstance abusers has not been adequately assessed.
-
2 DOSAGE AND ADMINISTRATION
The recommended dose of acamprosate calcium delayed-release tablets is two 333 mg tablets (each dose should total 666 mg) taken three times daily. A lower dose may be effective in some patients.
Although dosing may be done without regard to meals, dosing with meals was employed during clinical trials and is suggested in those patients who regularly eat three meals daily.
Treatment with acamprosate calcium delayed-release tablets should be initiated as soon as possible after the period of alcohol withdrawal, when the patient has achieved abstinence, and should be maintained if the patient relapses. Acamprosate calcium delayed-release tablets should be used as part of a comprehensive psychosocial treatment program.
2.1 Dosage in Renal Impairment
For patients with moderate renal impairment (creatinine clearance of 30 to 50 mL/min), a starting dose of one 333 mg tablet taken three times daily is recommended. Acamprosate calcium delayed-release tablets are contraindicated in patients with severe renal impairment (creatinine clearance of ≤30 mL/min) [see Contraindications (4.2), Warnings and Precautions (5.1), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity to Acamprosate Calcium
Acamprosate calcium is contraindicated in patients who previously have exhibited hypersensitivity to acamprosate calcium or any of its components.
4.2 Severe Renal Impairment
Acamprosate calcium is contraindicated in patients with severe renal impairment (creatinine clearance of ≤30 mL/min) [see Dosage and Administration (2.1), Warnings and Precautions (5.1), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
Treatment with acamprosate calcium in patients with moderate renal impairment (creatinine clearance of 30 to 50 mL/min) requires a dose reduction [see Dosage and Administration (2.1)]. Acamprosate calcium is contraindicated in patients with severe renal impairment (creatinine clearance of ≤30 mL/min) [see Dosage and Administration (2.1), Contraindications (4.2), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
5.2 Suicidality and Depression
In controlled clinical trials of acamprosate calcium, adverse events of a suicidal nature (suicidal ideation, suicide attempts, completed suicides) were infrequent overall, but were more common in acamprosate calcium-treated patients than in patients treated with placebo (1.4% vs. 0.5% in studies of 6 months or less; 2.4% vs. 0.8% in year-long studies). Completed suicides occurred in 3 of 2272 (0.13%) patients in the pooled acamprosate group from all controlled studies and 2 of 1962 patients (0.10%) in the placebo group. Adverse events coded as "depression" were reported at similar rates in acamprosate calcium-treated and placebo-treated patients. Although many of these events occurred in the context of alcohol relapse, and the interrelationship between alcohol dependence, depression and suicidality is well-recognized and complex, no consistent pattern of relationship between the clinical course of recovery from alcoholism and the emergence of suicidality was identified. Alcohol-dependent patients, including those patients being treated with acamprosate calcium, should be monitored for the development of symptoms of depression or suicidal thinking. Families and caregivers of patients being treated with acamprosate calcium should be alerted to the need to monitor patients for the emergence of symptoms of depression or suicidality, and to report such symptoms to the patient's health care provider.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Clinically significant serious adverse reactions associated with acamprosate calcium described elsewhere in labeling include suicidality and depression and acute kidney failure [see Warnings and Precautions (5.2),and Adverse Reactions (6.2)].
The adverse event data described below reflect the safety experience in over 7000 patients exposed to acamprosate calcium for up to one year, including over 2000 acamprosate calcium-exposed patients who participated in placebo-controlled trials.
Adverse Events Leading to Discontinuation
In placebo-controlled trials of 6 months or less, 8% of acamprosate calcium-treated patients discontinued treatment due to an adverse event, as compared to 6% of patients treated with placebo. In studies longer than 6 months, the discontinuation rate due to adverse events was 7% in both the placebo-treated and the acamprosate calcium-treated patients. Only diarrhea was associated with the discontinuation of more than 1% of patients (2% of acamprosate calcium-treated vs. 0.7% of placebo-treated patients). Other events, including nausea, depression, and anxiety, while accounting for discontinuation in less than 1% of patients, were nevertheless more commonly cited in association with discontinuation in acamprosate calcium-treated patients than in placebo-treated patients.
Common Adverse Events Reported in Controlled Trials
Common adverse events were collected spontaneously in some controlled studies and using a checklist in other studies. The overall profile of adverse events was similar using either method. Table 1 shows those events that occurred in any acamprosate calcium treatment group at a rate of 3% or greater and greater than the placebo group in controlled clinical trials with spontaneously reported adverse events. The reported frequencies of adverse events represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse event of the type listed, without regard to the causal relationship of the events to the drug.
Table 1. Events Occurring at a Rate of at Least 3% and Greater than Placebo in any Acamprosate Calcium Treatment Group in Controlled Clinical Trials with Spontaneously Reported Adverse Events. - * includes 258 patients treated with acamprosate calcium 2000 mg/day, using a different dosage strength and regimen.
- † includes all patients in the first two columns as well as 83 patients treated with acamprosate calcium 3000 mg/day, using a different dosage strength and regimen.
- ‡ includes events coded as “fracture” by sponsor;
- § includes events coded as “nervousness” by sponsor
Body System/ Preferred Term
Number of Patients (%) with Events
Acamprosate Calcium
1332 mg/day
Acamprosate Calcium
1998 mg/day*
Acamprosate Calcium
Pooled†
Placebo
Number of patients in
Treatment Group
397
1539
2019
1706
Number (%) of patients with an AE
248 (62%)
910 (59%)
1231 (61%)
955 (56%)
Body as a Whole
121 (30%)
513 (33%)
685 (34%)
517 (30%)
Accidental Injury‡
17 (4%)
44 (3%)
70 (3%)
52 (3%)
Asthenia
29 (7%)
79 (5%)
114 (6%)
93 (5%)
Pain
6 (2%)
56 (4%)
65 (3%)
55 (3%)
Digestive System
85 (21%)
440 (29%)
574 (28%)
344 (20%)
Anorexia
20 (5%)
35 (2%)
57 (3%)
44 (3%)
Diarrhea
39 (10%)
257 (17%)
329 (16%)
166 (10%)
Flatulence
4 (1%)
55 (4%)
63 (3%)
28 (2%)
Nausea
11 (3%)
69 (4%)
87 (4%)
58 (3%)
Nervous System
150 (38%)
417 (27%)
598 (30%)
500 (29%)
Anxiety§
32 (8%)
80 (5%)
118 (6%)
98 (6%)
Depression
33 (8%)
63 (4%)
102 (5%)
87 (5%)
Dizziness
15 (4%)
49 (3%)
67 (3%)
44 (3%)
Dry mouth
13 (3%)
23 (1%)
36 (2%)
28 (2%)
Insomnia
34 (9%)
94 (6%)
137 (7%)
121 (7%)
Paresthesia
11 (3%)
29 (2%)
40 (2%)
34 (2%)
Skin and Appendages
26 (7%)
150 (10%)
187 (9%)
169 (10%)
Pruritus
12 (3%)
68 (4%)
82 (4%)
58 (3%)
Sweating
11 (3%)
27 (2%)
40 (2%)
39 (2%)
Concomitant Therapies
In clinical trials, the safety profile in subjects treated with acamprosate calcium concomitantly with anxiolytics, hypnotics and sedatives (including benzodiazepines), or non-opioid analgesics was similar to that of subjects taking placebo with these concomitant medications. Patients taking acamprosate calcium concomitantly with antidepressants more commonly reported both weight gain and weight loss, compared with patients taking either medication alone.
Other Events Observed During the Premarketing Evaluation of Acamprosate Calcium
Following is a list of terms that reflect treatment-emergent adverse events reported by patients treated with acamprosate calcium in 20 clinical trials (4461 patients treated with acamprosate calcium, 3526 of whom received the maximum recommended dose of 1998 mg/day for up to one year in duration). This listing does not include those events already listed above; events for which a drug cause was considered remote; event terms which were so general as to be uninformative; and events reported only once which were not likely to be acutely life-threatening.
Events are further categorized by body system and listed in order of decreasing frequency according to the following definitions: frequent adverse events are those occurring in at least 1/100 patients (only those not already listed in the summary of adverse events in controlled trials appear in this listing); infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in fewer than 1/1000 patients.
Body as a Whole – Frequent: headache, abdominal pain, back pain, infection, flu syndrome, chest pain, chills, suicide attempt; Infrequent: fever, intentional overdose, malaise, allergic reaction, abscess, neck pain, hernia, intentional injury; Rare: ascites, face edema, photosensitivity reaction, abdomen enlarged, sudden death.
Cardiovascular System – Frequent: palpitation, syncope; Infrequent: hypotension, tachycardia, hemorrhage, angina pectoris, migraine, varicose vein, myocardial infarct, phlebitis, postural hypotension; Rare: heart failure, mesenteric arterial occlusion, cardiomyopathy, deep thrombophlebitis, shock.
Digestive System – Frequent: vomiting, dyspepsia, constipation, increased appetite; Infrequent: liver function tests abnormal, gastroenteritis, gastritis, dysphagia, eructation, gastrointestinal hemorrhage, pancreatitis, rectal hemorrhage, liver cirrhosis, esophagitis, hematemesis, nausea and vomiting, hepatitis; Rare: melena, stomach ulcer, cholecystitis, colitis, duodenal ulcer, mouth ulceration, carcinoma of liver.
Endocrine System – Rare: goiter, hypothyroidism.
Hemic and Lymphatic System – Infrequent: anemia, ecchymosis, eosinophilia, lymphocytosis, thrombocytopenia; Rare: leukopenia, lymphadenopathy, monocytosis.
Metabolic and Nutritional Disorders – Frequent – peripheral edema, weight gain; Infrequent: weight loss, hyperglycemia, SGOT increased, SGPT increased, gout, thirst, hyperuricemia, diabetes mellitus, avitaminosis, bilirubinemia; Rare: alkaline phosphatase increased, creatinine increased, hyponatremia, lactic dehydrogenase increased.
Musculoskeletal System – Frequent – myalgia, arthralgia; Infrequent: leg cramps; Rare: rheumatoid arthritis, myopathy.
Nervous System – Frequent –somnolence, libido decreased, amnesia, thinking abnormal, tremor, vasodilatation, hypertension; Infrequent: convulsion, confusion, libido increased, vertigo, withdrawal syndrome, apathy, suicidal ideation, neuralgia, hostility, agitation, neurosis, abnormal dreams, hallucinations, hypesthesia; Rare: alcohol craving, psychosis, hyperkinesia, twitching, depersonalization, increased salivation, paranoid reaction, torticollis, encephalopathy, manic reaction.
Respiratory System – Frequent: rhinitis, cough increased, dyspnea, pharyngitis, bronchitis; Infrequent: asthma, epistaxis, pneumonia; Rare: laryngismus, pulmonary embolus.
Skin and Appendages – Frequent: rash; Infrequent: acne, eczema, alopecia, maculopapular rash, dry skin, urticaria, exfoliative dermatitis, vesiculobullous rash; Rare: psoriasis.
Special Senses – Frequent: abnormal vision, taste perversion; Infrequent: tinnitus, amblyopia, deafness; Rare: ophthalmitis, diplopia, photophobia.
Urogenital System – Frequent: impotence; Infrequent – metrorrhagia, urinary frequency, urinary tract infection, sexual function abnormal, urinary incontinence, vaginitis; Rare: kidney calculus, abnormal ejaculation, hematuria, menorrhagia, nocturia, polyuria, urinary urgency.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of acamprosate calcium. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
-
7 DRUG INTERACTIONS
Acamprosate does not affect the pharmacokinetics of alcohol. The pharmacokinetics of acamprosate are not affected by alcohol, diazepam, or disulfiram, and clinically important interactions between naltrexone and acamprosate were not observed [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Teratogenic effects: Acamprosate calcium has been shown to be teratogenic in rats when given in doses that are approximately equal to the human dose (on a mg/m2 basis) and in rabbits when given in doses that are approximately 3 times the human dose (on a mg/m2 basis). Acamprosate calcium produced a dose-related increase in the number of fetuses with malformations in rats at oral doses of 300 mg/kg/day or greater (approximately equal to the maximum recommended human daily (MRHD) oral dose on a mg/m2 basis). The malformations included hydronephrosis, malformed iris, retinal dysplasia, and retroesophageal subclavian artery. No findings were observed at an oral dose of 50 mg/kg/day (approximately one-fifth the MRHD oral dose on a mg/m2 basis). An increased incidence of hydronephrosis was also noted in Burgundy Tawny rabbits at oral doses of 400 mg/kg/day or greater (approximately 3 times the MRHD oral dose on a mg/m2 basis). No developmental effects were observed in New Zealand white rabbits at oral doses up to 1000 mg/kg/day (approximately 8 times the MRHD oral dose on a mg/m2 basis). The findings in animals should be considered in relation to known adverse developmental effects of ethyl alcohol, which include the characteristics of fetal alcohol syndrome (craniofacial dysmorphism, intrauterine and postnatal growth retardation, retarded psychomotor and intellectual development) and milder forms of neurological and behavioral disorders in humans. There are no adequate and well controlled studies in pregnant women. Acamprosate calcium should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic effects: A study conducted in pregnant mice that were administered acamprosate calcium by the oral route starting on Day 15 of gestation through the end of lactation on postnatal day 28 demonstrated an increased incidence of still-born fetuses at doses of 960 mg/kg/day or greater (approximately 2 times the MRHD oral dose on a mg/m2 basis). No effects were observed at a dose of 320 mg/kg/day (approximately one-half the MRHD dose on a mg/m2 basis).
8.2 Labor and Delivery
The potential for acamprosate calcium to affect the duration of labor and delivery is unknown.
8.3 Nursing Mothers
In animal studies, acamprosate was excreted in the milk of lactating rats dosed orally with acamprosate calcium. The concentration of acamprosate in milk compared to blood was 1.3:1. It is not known whether acamprosate is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when acamprosate calcium is administered to a nursing woman.
8.4 Pediatric Use
The safety and efficacy of acamprosate calcium have not been established in the pediatric population.
8.5 Geriatric Use
Forty-one of the 4234 patients in double-blind, placebo-controlled, clinical trials of acamprosate calcium were 65 years of age or older, while none were 75 years of age or over. There were too few patients in the ≥65 age group to evaluate any differences in safety or effectiveness for geriatric patients compared to younger patients.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Clinical Pharmacology (12.3), Adverse Reactions (6.1), and Dosage and Administration (2.1)].
8.6 Renal Impairment
Acamprosate calcium is contraindicated in patients with severe renal impairment (creatinine clearance of ≤30 mL/min) [see Dosage and Administration (2.1), Contraindications (4.2), Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
In all reported cases of acute overdosage with acamprosate calcium (total reported doses of up to 56 grams of acamprosate calcium), the only symptom that could be reasonably associated with acamprosate calcium was diarrhea. Hypercalcemia has not been reported in cases of acute overdose. A risk of hypercalcemia should be considered in chronic overdosage only. Treatment of overdose should be symptomatic and supportive.
-
11 DESCRIPTION
Acamprosate calcium is supplied in an enteric-coated tablet for oral administration. Acamprosate calcium is a synthetic compound with a chemical structure similar to that of the endogenous amino acid homotaurine, which is a structural analogue of the amino acid neurotransmitter γ-aminobutyric acid and the amino acid neuromodulator taurine. Its chemical name is calcium acetylaminopropane sulfonate. Its chemical formula is C10H20N2O8S2Ca and molecular weight is 400.5. Its structural formula is:
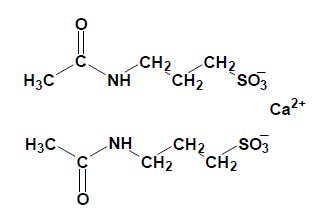
Acamprosate calcium is a white or almost white powder. It is freely soluble in water, practically insoluble in alcohol and in methylene chloride.
Each acamprosate calcium delayed-release tablet intended for oral administration contains 333 mg of acamprosate calcium equivalent to 300 mg of acamprosate. In addition, each tablet contains the following inactive ingredients: colloidal anhydrous silica, methacrylic acid copolymer type c, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, sodium bicarbonate, sodium lauryl sulfate, sodium starch glycolate and talc. Sulfites were used in the synthesis of the drug substance and traces of residual sulfites may be present in the drug product.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of acamprosate in maintenance of alcohol abstinence is not completely understood. Chronic alcohol exposure is hypothesized to alter the normal balance between neuronal excitation and inhibition. In vitro and in vivo studies in animals
have provided evidence to suggest acamprosate may interact with glutamate and GABA neurotransmitter systems centrally, and has led to the hypothesis that acamprosate restores this balance.
12.2 Pharmacodynamics
Pharmacodynamic studies have shown that acamprosate calcium reduces alcohol intake in alcohol-dependent animals in a dose-dependent manner and that this effect appears to be specific to alcohol and the mechanisms of alcohol dependence.
Acamprosate calcium has negligible observable central nervous system (CNS) activity in animals outside of its effects on alcohol dependence, exhibiting no anticonvulsant, antidepressant, or anxiolytic activity.
The administration of acamprosate calcium is not associated with the development of tolerance or dependence in animal studies. Acamprosate calcium did not produce any evidence of withdrawal symptoms in patients in clinical trials at therapeutic doses. Post marketing data, collected retrospectively outside the U.S. have provided no evidence of acamprosate calcium abuse or dependence.
Acamprosate calcium is not known to cause alcohol aversion and does not cause a disulfiram-like reaction as a result of ethanol ingestion.
12.3 Pharmacokinetics
The absolute bioavailability of acamprosate calcium after oral administration is about 11%. Steady-state plasma concentrations of acamprosate are reached within 5 days of dosing. Steady-state peak plasma concentrations after acamprosate calcium doses of 2 x 333 mg tablets three times daily average 350 ng/mL and occur at 3-8 hours post-dose. Coadministration of acamprosate calcium with food decreases bioavailability as measured by Cmax and AUC, by approximately 42% and 23%, respectively. The food effect on absorption is not clinically significant and no adjustment of dose is necessary.
Distribution
The volume of distribution for acamprosate following intravenous administration is estimated to be 72-109 liters (approximately 1 L/kg). Plasma protein binding of acamprosate is negligible.
Metabolism
Acamprosate does not undergo metabolism.
Elimination
After oral dosing of 2 x 333 mg of acamprosate calcium, the terminal half-life ranges from approximately 20-33 hours. Following oral administration of acamprosate calcium, the major route of excretion is via the kidneys as acamprosate.
Special Populations
Gender: Acamprosate calcium does not exhibit any significant pharmacokinetic differences between male and female subjects.
Age: The pharmacokinetics of acamprosate calcium have not been evaluated in a geriatric population. However, since renal function diminishes in elderly patients and acamprosate is excreted unchanged in urine, acamprosate plasma concentrations are likely to be higher in the elderly population compared to younger adults.
Pediatrics: The pharmacokinetics of acamprosate calcium have not been evaluated in a pediatric population.
Renal Impairment: Peak plasma concentrations after administration of a single dose of 2 x 333 mg acamprosate calcium delayed-release tablets to patients with moderate or severe renal impairment were about 2-fold and 4-fold higher, respectively, compared to healthy subjects. Similarly, elimination half-life was about 1.8-fold and 2.6-fold longer, respectively, compared to healthy subjects. There is a linear relationship between creatinine clearance values and total apparent plasma clearance, renal clearance and plasma half-life of acamprosate. A dose of 1 x 333 mg acamprosate calcium delayed-release tablets, three times daily, is recommended in patients with moderate renal impairment (creatinine clearance of 30-50 mL/min, [see Use in Specific Populations (8.6)].
Acamprosate calcium is contraindicated in patients with severe renal impairment (creatinine clearance of ≤30 mL/min) [see Dosage and Administration (2.1),Contraindications (4.2), Warnings and Precautions (5.1), and Use in Specific Populations (8.6)].
Hepatic Impairment: Acamprosate is not metabolized by the liver and the pharmacokinetics of acamprosate calcium are not altered in patients with mild to moderate hepatic impairment (groups A and B of the Child-Pugh classification). No adjustment of dosage is recommended in such patients.
Alcohol-dependent subjects: A cross-study comparison of acamprosate calcium at doses of 2 x 333 mg three times daily indicated similar pharmacokinetics between alcohol-dependent subjects and healthy subjects.
Drug-Drug Interactions
Acamprosate had no inducing potential on the cytochrome CYP1A2 and 3A4 systems, and in vitro inhibition studies suggest that acamprosate does not inhibit in vivo metabolism mediated by cytochrome CYP1A2, 2C9, 2C19, 2D6, 2E1, or 3A4. The pharmacokinetics of acamprosate calcium were unaffected when co-administered with alcohol, disulfiram or diazepam. Similarly, the pharmacokinetics of ethanol, diazepam and nordiazepam, imipramine and desipramine, naltrexone and 6-beta naltrexol were unaffected following co-administration with acamprosate calcium. However, co-administration of acamprosate calcium with naltrexone led to a 33% increase in the Cmax and a 25% increase in the AUC of acamprosate. No adjustment of dosage is recommended in such patients.
-
13 NONCLINICAL TOXICOLOGY
13.3 Carcinogenesis, Mutagenesis, Impairment of Fertility
Dietary administration of acamprosate calcium for 2 years to Sprague-Dawley rats at doses of 25, 100 and 400 mg/kg/day (up to 3 times the maximum recommended human daily (MRHD) oral dose on an AUC basis) and CD-1 mice at doses of 400, 1200 and 3600 mg/kg/day (up to 25 times the MRHD on an AUC basis) showed no evidence of increased tumor incidence.
Acamprosate calcium was negative in all genetic toxicology studies conducted. Acamprosate calcium demonstrated no evidence of genotoxicity in an in vitro bacterial reverse point mutation assay (Ames assay) or an in vitro mammalian cell gene mutation test using Chinese Hamster Lung V79 cells. No clastogenicity was observed in an in vitro chromosomal aberration assay in human lymphocytes and no chromosomal damage detected in an in vivo mouse micronucleus assay.
Acamprosate calcium had no effect on fertility after treatment for 70 days prior to mating in male rats and for 14 days prior to mating, throughout mating, gestation and lactation in female rats at doses up to 1000 mg/kg/day (approximately 4 times the MRHD oral dose on a mg/m2 basis). In mice, acamprosate calcium administered orally for 60 days prior to mating and throughout gestation in females at doses up to 2400 mg/kg/day (approximately 5 times the MRHD oral dose on a mg/m2 basis) had no effect on fertility.
-
14 CLINICAL STUDIES
The efficacy of acamprosate calcium in the maintenance of abstinence was supported by three clinical studies involving a total of 998 patients who were administered at least one dose of acamprosate calcium or placebo as an adjunct to psychosocial therapy. Each study was a double-blind, placebo-controlled trial in alcohol-dependent patients who had undergone inpatient detoxification and were abstinent from alcohol on the day of randomization. Study durations ranged from 90 days to 360 days. Acamprosate calcium proved superior to placebo in maintaining abstinence, as indicated by a greater percentage of subjects being assessed as continuously abstinent throughout treatment.
In a fourth study, the efficacy of acamprosate calcium was evaluated in alcoholics, including patients with a history of polysubstance abuse and patients who had not undergone detoxification and were not required to be abstinent at baseline. This study failed to demonstrate superiority of acamprosate calcium over placebo.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Acamprosate Calcium Delayed-release Tablets, 333 mg are white to off-white, round, biconvex, beveled edge, enteric coated tablets, debossed with '569' on one side and plain on the other side and are supplied as follows:
NDC: 68382-569-06 in bottles of 30 tablets
NDC: 68382-569-16 in bottles of 90 tablets
NDC: 68382-569-01 in bottles of 100 tablets
NDC: 68382-569-28 in bottles of 180 tablets
NDC: 68382-569-05 in bottles of 500 tablets
NDC: 68382-569-10 in bottles of 1000 tablets
Storage and Handling
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight container (USP).
-
17 PATIENT COUNSELING INFORMATION
17.1 Information for Patients
Physicians are advised to discuss the following issues with patients for whom they prescribe acamprosate calcium delayed-release tablets.
Renal Impairment
A lower dose is recommended for patients with moderate renal impairment. Acamprosate calcium delayed-release tablets are contraindicated in patients with severe renal impairment (creatinine clearance of ≤30 mL/min) [see Dosage and Administration (2.1), Contraindications (4.2), Warnings and Precautions (5.1) and Use in Specific Populations (8.6)].
Suicidality and Depression
Families and caregivers of patients being treated with acamprosate calcium delayed-release tablets should be alerted to the need to monitor patients for the emergence of symptoms of depression or suicidality, and to report such symptoms to the patient's health care provider [see Warnings and Precautions (5.2)].
Alcohol Withdrawal
Use of acamprosate calcium delayed-release tablets does not eliminate or diminish withdrawal symptoms [see Warnings and Precautions (5.3)].
Pregnancy and Breast Feeding
- Advise patients to notify their physician if they become pregnant or intend to become pregnant during therapy.
- Advise patients to notify their physician if they are breast-feeding.
- Advise patients to continue acamprosate calcium delayed-release tablets therapy as directed, even in the event of relapse and remind them to discuss any renewed drinking with their physicians.
- Advise patients that acamprosate calcium delayed-release tablets has been shown to help maintain abstinence only when used as a part of a treatment program that includes counseling and support.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Manufactured by:
Cadila Healthcare Ltd.
Baddi, India
Distributed by:
Zydus Pharmaceuticals USA Inc.
Pennington, NJ 08534
Revision Date: 01/06/16
Rev.: 06/16
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 68382-569-28 in bottles of 180 tablets
Acamprosate Calcium Delayed-release Tablets
333 mg
Rx only
Zydus
-
INGREDIENTS AND APPEARANCE
ACAMPROSATE CALCIUM
acamprosate calcium tablet, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68382-569 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACAMPROSATE CALCIUM (UNII: 59375N1D0U) (ACAMPROSATE - UNII:N4K14YGM3J) ACAMPROSATE CALCIUM 333 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID (UNII: 1CS02G8656) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) POVIDONE K90 (UNII: RDH86HJV5Z) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND Size 10mm Flavor Imprint Code 569 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68382-569-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2017 2 NDC: 68382-569-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2017 3 NDC: 68382-569-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2017 4 NDC: 68382-569-28 180 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2017 5 NDC: 68382-569-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2017 6 NDC: 68382-569-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205995 06/01/2017 Labeler - Zydus Pharmaceuticals (USA) Inc. (156861945)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.