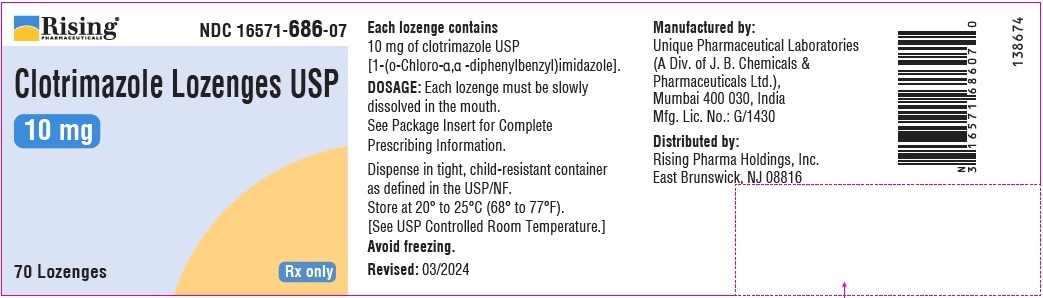
CLOTRIMAZOLE by Rising Pharma Holdings, Inc. / Unique Pharmaceutical Laboratories CLOTRIMAZOLE lozenge
CLOTRIMAZOLE by
Drug Labeling and Warnings
CLOTRIMAZOLE by is a Prescription medication manufactured, distributed, or labeled by Rising Pharma Holdings, Inc., Unique Pharmaceutical Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each Clotrimazole lozenge USP contains 10 mg clotrimazole USP [1-(o-chloro-α,α-diphenylbenzyl) imidazole], a synthetic antifungal agent, for topical use in the mouth.
Structural Formula:
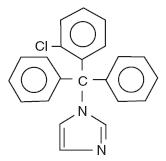
Chemical Formula: C22H17ClN2
The lozenge dosage form is a large, slowly dissolving lozenge containing 10 mg of clotrimazole USP dispersed in croscarmellose sodium, dextrates, magnesium stearate, microcrystalline cellulose and povidone.
-
CLINICAL PHARMACOLOGY
Clotrimazole is a broad-spectrum antifungal agent that inhibits the growth of pathogenic yeasts by altering the permeability of cell membranes. The action of clotrimazole is fungistatic at concentrations of drug up to 20 mcg/mL and may be fungicidal in vitro against Candida albicans and other species of the genus Candida at higher concentrations. No single-step or multiple-step resistance to clotrimazole has developed during successive passages of Candida albicans in the laboratory; however, individual organism tolerance has been observed during successive passages in the laboratory. Such in vitro tolerance has resolved once the organism has been removed from the antifungal environment.
After oral administration of a 10 mg clotrimazole lozenge to healthy volunteers, concentrations sufficient to inhibit most species of Candida persist in saliva for up to three hours following the approximately 30 minutes needed for a lozenge to dissolve. The long term persistence of drug in saliva appears to be related to the slow release of clotrimazole from the oral mucosa to which the drug is apparently bound. Repetitive dosing at three hour intervals maintains salivary levels above the minimum inhibitory concentrations of most strains of Candida; however, the relationship between in vitro susceptibility of pathogenic fungi to clotrimazole and prophylaxis or cure of infections in humans has not been established.
In another study, the mean serum concentrations were 4.98 ± 3.7 and 3.23 ± 1.4 nanograms/mL of clotrimazole at 30 and 60 minutes, respectively, after administration as a lozenge.
-
INDICATIONS AND USAGE
Clotrimazole Lozenges are indicated for the local treatment of oropharyngeal candidiasis. The diagnosis should be confirmed by a KOH smear and/or culture prior to treatment.
Clotrimazole Lozenges are also indicated prophylactically to reduce the incidence of oropharyngeal candidiasis in patients immunocompromised by conditions that include chemotherapy, radiotherapy, or steroid therapy utilized in the treatment of leukemia, solid tumors, or renal transplantation. There are no data from adequate and well-controlled trials to establish the safety and efficacy of this product for prophylactic use in patients immunocompromised by etiologies other than those listed in the previous sentence. (See DOSAGE AND ADMINISTRATION.)
- CONTRAINDICATIONS
- WARNING
-
PRECAUTIONS
Abnormal liver function tests have been reported in patients treated with clotrimazole lozenges; elevated SGOT levels were reported in about 15% of patients in the clinical trials. In most cases the elevations were minimal and it was often impossible to distinguish effects of clotrimazole from those of other therapy and the underlying disease (malignancy in most cases). Periodic assessment of hepatic function is advisable particularly in patients with pre-existing hepatic impairment.
Since patients must be instructed to allow each lozenge to dissolve slowly in the mouth in order to achieve maximum effect of the medication, they must be of such an age and physical and/or mental condition to comprehend such instructions.
Carcinogenesis
An 18 month dosing study with clotrimazole in rats has not revealed any carcinogenic effect.
Use In Pregnancy
Pregnancy Category C:
Clotrimazole has been shown to be embryotoxic in rats and mice when given in doses 100 times the adult human dose (in mg/kg), possibly secondary to maternal toxicity. The drug was not teratogenic in mice, rabbits, and rats when given in doses up to 200, 180, and 100 times the human dose.
Clotrimazole given orally to mice from nine weeks before mating through weaning at a dose 120 times the human dose was associated with impairment of mating, decreased number of viable young, and decreased survival to weaning. No effects were observed at 60 times the human dose. When the drug was given to rats during a similar time period at 50 times the human dose, there was a slight decrease in the number of pups per litter and decreased pup viability.
There are no adequate and well controlled studies in pregnant women. Clotrimazole lozenges should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pediatric Use
Safety and effectiveness of clotrimazole in children below the age of 3 years have not been established; therefore, its use in such patients is not recommended.
The safety and efficacy of the prophylactic use of clotrimazole lozenges in children have not been established.
Geriatric Use
Clinical studies of clotrimazole did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
ADVERSE REACTIONS
Abnormal liver function tests have been reported in patients treated with clotrimazole lozenges; elevated SGOT levels were reported in about 15% of patients in the clinical trials (See PRECAUTIONS).
Nausea, vomiting, unpleasant mouth sensations and pruritus have also been reported with the use of the lozenge.
To report SUSPECTED ADVERSE REACTIONS, contact Rising Pharma Holdings, Inc. at 1-844-874-7464 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
- OVERDOSAGE
- DRUG ABUSE AND DEPENDENCE
-
DOSAGE AND ADMINISTRATION
Clotrimazole Lozenges are administered only as a lozenge that must be slowly dissolved in the mouth. The recommended dose is one lozenge five times a day for fourteen consecutive days. Only limited data are available on the safety and effectiveness of the clotrimazole lozenge after prolonged administration; therefore, therapy should be limited to short term use, if possible.
For prophylaxis to reduce the incidence of oropharyngeal candidiasis in patients immunocompromised by conditions that include chemotherapy, radiotherapy, or steroid therapy utilized in the treatment of leukemia, solid tumors, or renal transplantation, the recommended dose is one lozenge three times daily for the duration of chemotherapy or until steroids are reduced to maintenance levels.
-
HOW SUPPLIED
Clotrimazole Lozenges USP
10 mg lozenge is supplied as white to off white, round, flat face beveled edge lozenge debossed with “227” on one side and “P” on the other side.
NDC: 16571-686-07: Bottle of 70 Lozenges with child-resistant closure.
NDC: 16571-686-14: Bottle of 140 Lozenges with child-resistant closure.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLOTRIMAZOLE
clotrimazole lozengeProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 16571-686 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOTRIMAZOLE (UNII: G07GZ97H65) (CLOTRIMAZOLE - UNII:G07GZ97H65) CLOTRIMAZOLE 10 mg Inactive Ingredients Ingredient Name Strength DEXTRATES (UNII: G263MI44RU) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POVIDONE K30 (UNII: U725QWY32X) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND Size 16mm Flavor Imprint Code 227;P Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16571-686-07 70 in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2024 2 NDC: 16571-686-14 140 in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215641 05/01/2024 Labeler - Rising Pharma Holdings, Inc. (116880195) Establishment Name Address ID/FEI Business Operations Unique Pharmaceutical Laboratories 650434645 manufacture(16571-686)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.