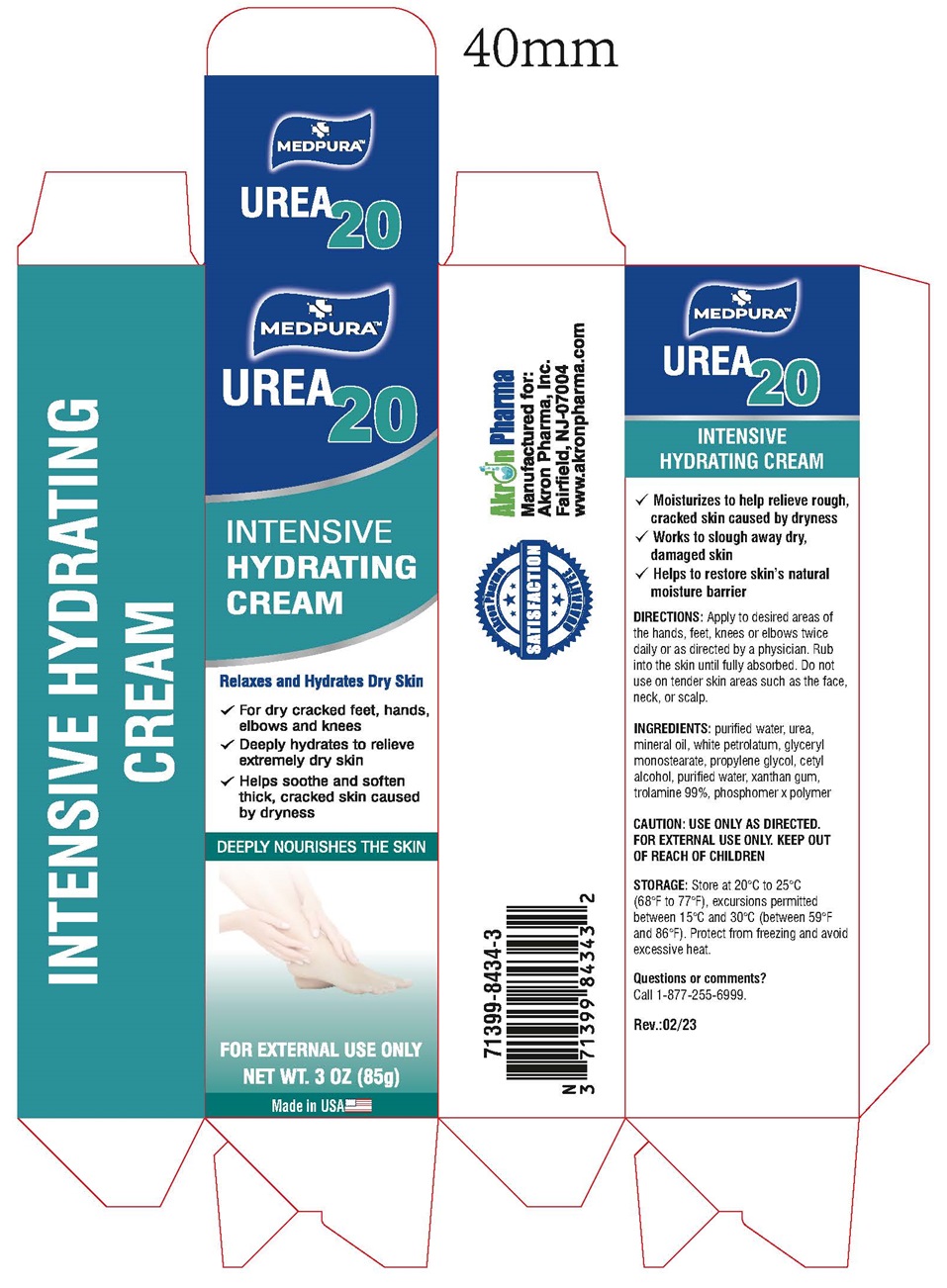
MEDPURA Urea 20Intensive Hydrating Cream
Urea 20 by
Drug Labeling and Warnings
Urea 20 by is a Other medication manufactured, distributed, or labeled by Akron Pharma Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
UREA 20- urea cream
Akron Pharma Inc.
----------
MEDPURA Urea 20
Intensive Hydrating Cream
INTENSIVE HYDRATING CREAM
- Moisturizes to help relieve rough, cracked skin caused by dryness
- Works to slough away dry, damaged skin
- Helps to restore skin’s natural moisture barrier
INGREDIENTS: purified water, urea, mineral oil, white petrolatum, glyceryl monostearate, propylene glycol, cetyl alcohol, purified water, xanthan gum, trolamine 99%, phosphomer x polymer.
| UREA 20
urea cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Akron Pharma Inc. (067878881) |
Revised: 12/2023
Document Id: 2a554e89-1a77-439c-8caa-cf038a90ec81
Set id: 982e54c8-7c17-44fe-b05b-d3df03c45493
Version: 1
Effective Time: 20231208
Trademark Results [Urea 20]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 UREA 20 88387619 5870273 Live/Registered |
Scientific Solutions Global LLC 2019-04-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.