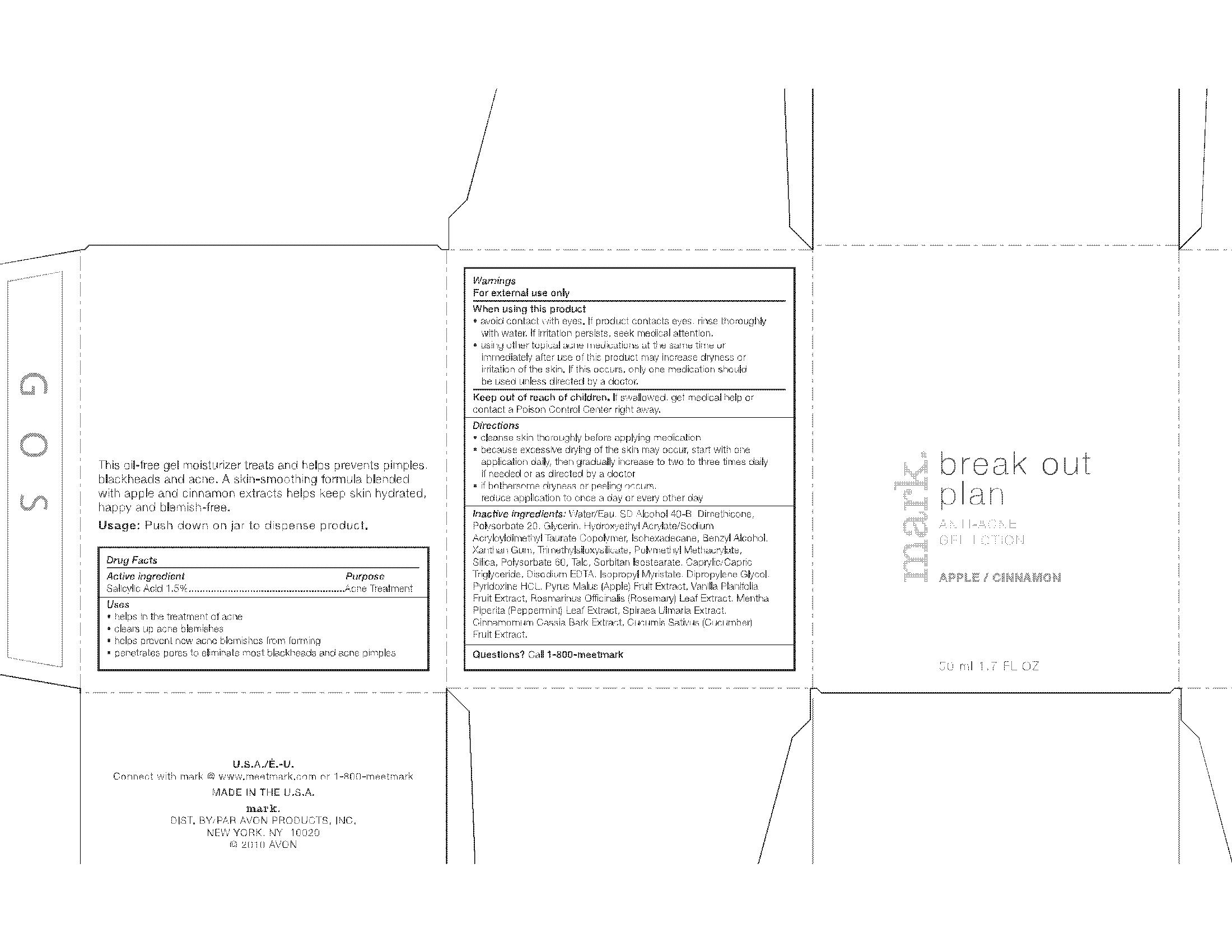
MARK.- salicylic acid gel
MARK. by
Drug Labeling and Warnings
MARK. by is a Otc medication manufactured, distributed, or labeled by Avon Products Inc., Avon products, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
When using this product
- avoid contact with eyes. If product contacts eyes, rinse thoroughly with water. If irritation persists, seek medical attention
- using other topical acne products at the same time or immediately after use of this product may increase dryness or irritation of the skin. If this occurs, only one product should be used unless directed by a doctor.
-
DOSAGE & ADMINISTRATION
Directions
- cleanse skin thoroughly before applying medication
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two to three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
-
INACTIVE INGREDIENT
Inactive/Non-medicinal ingredients
WATER/EAU
SD ALCOHOL 40-B
DIMETHICONE
POLYSORBATE 20
GLYCERIN
PYRUS MALUS (APPLE) FRUIT EXTRACT
VANILLA PLANIFOLIA FRUIT EXTRACT
ROSMARINUS OFFICINALIS (ROSEMARY) LEAF EXTRACT
MENTHA PIPERITA (PEPPERMINT) LEAF EXTRACT
SPIRAEA ULMARIA EXTRACT
CINNAMOMUM CASSIA BARK EXTRACT
CUCUMIS SATIVUS (CUCUMBER) FRUIT EXTRACT
HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER
ISOHEXADECANE
XANTHAN GUM
TRIMETHYLSILOXYSILICATE
POLYMETHYL METHACRYLATE
SILICA
POLYSORBATE 60
TALC
SORBITAN ISOSTEARATE
CAPRYLIC/CAPRIC TRIGLYCERIDE
ISOPROPYL MYRISTATE
DIPROPYLENE GLYCOL
PYRIDOXINE HCL
DISODIUM EDTA
BENZYL ALCOHOL
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MARK. BREAK OUT PLAN
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10096-0195 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID .75 mL in 50 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10096-0195-1 50 mL in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 12/29/2009 Labeler - Avon Products Inc. (001468693) Establishment Name Address ID/FEI Business Operations Avon products, Inc. 005149471 manufacture
Trademark Results [MARK.]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MARK. 87374032 not registered Live/Pending |
AVON NA IP, LLC 2017-03-16 |
 MARK. 85505536 4201461 Dead/Cancelled |
AVON NA IP LLC 2011-12-29 |
 MARK. 85505520 4256483 Dead/Cancelled |
AVON NA IP LLC 2011-12-29 |
 MARK. 85505486 4252873 Dead/Cancelled |
AVON NA IP LLC 2011-12-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.