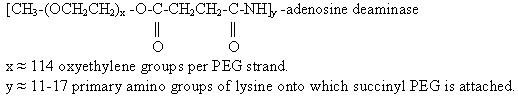ADAGEN® (pegademase bovine) Injection
Adagen by
Drug Labeling and Warnings
Adagen by is a Prescription medication manufactured, distributed, or labeled by Leadiant Biosciences, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ADAGEN- pegademase bovine injection, solution
Leadiant Biosciences, Inc.
----------
ADAGEN® (pegademase bovine) Injection
DESCRIPTION
ADAGEN® (pegademase bovine) Injection is a modified enzyme used for enzyme replacement therapy for the treatment of severe combined immunodeficiency disease (SCID) associated with a deficiency of adenosine deaminase.
ADAGEN® (pegademase bovine) Injection is supplied in an isotonic, pyrogen free, sterile solution, pH 7.2-7.4, for intramuscular injection only. The solution is clear and colorless. It is supplied in 1.5 mL single-dose vials.
The chemical name for ADAGEN® (pegademase bovine) Injection is (monomethoxypolyethylene glycol succinimidyl) 11-17-adenosine deaminase. It is a conjugate of numerous strands of monomethoxypolyethylene glycol (PEG), molecular weight 5,000, covalently attached to the enzyme adenosine deaminase (ADA). ADA (adenosine deaminase EC 3.5.4.4) used in the manufacture of ADAGEN® (pegademase bovine) Injection is derived from bovine intestine.
The structural formula of ADAGEN® (pegademase bovine) Injection is:
Each milliliter of ADAGEN® (pegademase bovine) Injection contains:
Pegademase bovine 250 units*
Monobasic sodium phosphate, USP 1.20 mg
Dibasic sodium phosphate, USP 5.58 mg
Sodium Chloride, USP 8.50 mg
Water for injection, USP q.s. to 1.0 mL
*One unit of activity is defined as the amount of ADA that converts 1μM of adenosine to inosine per minute at 25°C and pH 7.3.
CLINICAL PHARMACOLOGY
Severe Combined Immunodeficiency Disease Associated with ADA Deficiency
Severe combined immunodeficiency disease (SCID) associated with a deficiency of ADA is a rare, inherited, and often fatal disease. In the absence of the ADA enzyme, the purine substrates adenosine and 2′-deoxyadenosine accumulate, causing metabolic abnormalities that are directly toxic to lymphocytes.
The immune deficiency can be cured by bone marrow transplantation. When a suitable bone marrow donor is unavailable or when bone marrow transplantation fails, non-selective replacement of the ADA enzyme has been provided by periodic irradiated red blood cell transfusions. However, transmission of viral infections and iron overload are serious risks associated with irradiated red blood cell transfusions, and relatively few ADA deficient patients have benefitted from chronic transfusion therapy.
ADAGEN® (pegademase bovine) Injection provides specific and direct replacement of the deficient enzyme, but will not benefit patients with immunodeficiency due to other causes.
In patients with ADA deficiency, rigorous adherence to a schedule of ADAGEN® (pegademase bovine) Injection administration can eliminate the toxic metabolites of ADA deficiency and result in improved immune function. It is imperative that treatment with ADAGEN® (pegademase bovine) Injection be carefully monitored by measurement of the level of ADA activity in plasma. Monitoring of the level of deoxyadenosine triphosphate (dATP) in erythrocytes is also helpful in determining that the dose of ADAGEN® (pegademase bovine) Injection is adequate.
Actions
ADAGEN® (pegademase bovine) Injection provides specific replacement of the deficient enzyme.
In the absence of the enzyme ADA, the purine substrates adenosine, 2′-deoxyadenosine and their metabolites are toxic to lymphocytes. The direct action of ADAGEN® (pegademase bovine) Injection is the correction of these metabolic abnormalities. Improvement in immune function and diminished frequency of opportunistic infections compared with the natural history of combined immunodeficiency due to ADA deficiency only occurs after metabolic abnormalities are corrected. There is a lag between the correction of the metabolic abnormalities and improved immune function. This period of time is variable, and has been reported to be from a few weeks to as long as 6 months. In contrast to the natural history of combined immunodeficiency disease due to ADA deficiency, a trend toward diminished frequency of opportunistic infections and fewer complications of infections has occurred in patients receiving ADAGEN® (pegademase bovine) Injection.
Pharmacokinetics
The pharmacokinetics and biochemical effects of ADAGEN® (pegademase bovine) Injection have been studied in six children ranging in age from 6 weeks to 12 years with SCID associated with ADA deficiency.
After the intramuscular injection of ADAGEN® (pegademase bovine) Injection, peak plasma levels of ADA activity were reached 2 to 3 days following administration. The plasma elimination half-life of ADA following the administration of ADAGEN® (pegademase bovine) Injection was variable, even for the same child. The range was 3 to > 6 days. Following weekly injections of ADAGEN® (pegademase bovine) Injection at 15 U/kg, the average trough level of ADA activity in plasma was between 20 and 25 μmol/hr/mL.
Biochemical Effects
The changes in red blood cell deoxyadenosine nucleotide (dATP) and S-adenosylhomocysteine hydrolase (SAHase) have been evaluated. In patients with ADA deficiency, inadequate elimination of 2′-deoxyadenosine caused a marked elevation in dATP and a decrease in SAHase level in red blood cells. Prior to treatment with ADAGEN® (pegademase bovine) Injection, the levels of dATP in the red blood cells ranged from 0.056 to 0.899 μmol/mL of erythrocytes. After 2 months of maintenance treatment with ADAGEN® (pegademase bovine) Injection, the levels decreased to 0.007 to 0.015 μmol/mL. The normal value of dATP is below 0.001 μmol/mL. In the same period of time, the levels of SAHase increased from the pretreatment range of 0.09 to 0.22 nmol/hr/mg protein to a range of 2.37 to 5.16 nmol/hr/mg protein. The normal value for SAHase is 4.18 ± 1.9 nmol/hr/mg protein.
The optimal dosage and schedule of administration of ADAGEN® (pegademase bovine) Injection should be established for each patient, based on monitoring of plasma ADA activity levels (trough levels before maintenance injection), biochemical markers of ADA deficiency (primarily red cell dATP content), and parameters of immune function. Since improvement in immune function follows correction of metabolic abnormalities, maintenance dosage in individual patients should be aimed at achieving the following biochemical goals: 1) maintain plasma ADA activity (trough levels) in the range of 15-35 μmol/hr/mL (assayed at 37°C); and 2) decline in erythrocyte dATP to ≤ 0.005-0.015 µmol/mL packed erythrocytes, or ≤ 1% of the total erythrocyte adenine nucleotide (ATP + dATP) content, with a normal ATP level, as measured in a pre-injection sample.
In vitro immunologic data (lymphocyte response to mitogens and lymphocyte surface antigens) were obtained, but their clinical significance is unknown. Prior to treatment with ADAGEN® (pegademase bovine) Injection, immune status was significantly below normal, as indicated by < 10% of normal mitogen responses and circulating mononuclear cells bearing T-cell surface antigens. These parameters improved, though not always to normal, within 2 to 6 months of therapy.
INDICATIONS AND USAGE
ADAGEN® (pegademase bovine) Injection is indicated for enzyme replacement therapy for adenosine deaminase (ADA) deficiency in patients with severe combined immunodeficiency disease (SCID) who are not suitable candidates for – or who have failed – bone marrow transplantation. ADAGEN® (pegademase bovine) Injection is recommended for use in infants from birth or in children of any age at the time of diagnosis. ADAGEN® (pegademase bovine) Injection is not intended as a replacement for HLA identical bone marrow transplant therapy. ADAGEN® (pegademase bovine) Injection is also not intended to replace continued close medical supervision and the initiation of appropriate diagnostic tests and therapy (e.g., antibiotics, nutrition, oxygen, gammaglobulin) as indicated for intercurrent illnesses.
CONTRAINDICATIONS
There is no evidence to support the safety and efficacy of ADAGEN® (pegademase bovine) Injection as preparatory or support therapy for bone marrow transplantation. Since ADAGEN® (pegademase bovine) Injection is administered by intramuscular injection, it should be used with caution in patients with thrombocytopenia and should not be used if thrombocytopenia is severe.
PRECAUTIONS
General
Any laboratory or clinical indication of a decrease in potency of ADAGEN® (pegademase bovine) Injection should be reported immediately by telephone to Leadiant Biosciences, Inc. Telephone 1-866-792-5172.
There have been no reports of hypersensitivity reactions in patients who have been treated with ADAGEN® (pegademase bovine) Injection.
One of 12 patients showed an enhanced rate of clearance of plasma ADA activity after 5 months of therapy at 15 U/kg/week. Enhanced clearance was correlated with the appearance of an antibody that directly inhibited both unmodified ADA and ADAGEN® (pegademase bovine) Injection. Subsequently, the patient was treated with twice weekly intramuscular injections at an increased dose of 20 U/kg, or a total weekly dose of 40 U/kg. No adverse effects were observed at the higher dose and effective levels of plasma ADA were restored. After 4 months, the patient returned to a weekly dosage schedule of 20 U/kg and effective plasma levels have been maintained.
Appropriate care to protect immune deficient patients should be maintained until improvement in immune function has been documented. The degree of immune function improvement may vary from patient to patient and, therefore, each patient will require appropriate care consistent with immunologic status.
Laboratory Tests
The treatment of SCID associated with ADA deficiency with ADAGEN® (pegademase bovine) Injection should be monitored by measuring plasma ADA activity and red blood cell dATP levels.
Plasma ADA activity and red cell dATP should be determined prior to treatment. Once treatment with ADAGEN® (pegademase bovine) Injection has been initiated, a desirable range of plasma ADA activity (trough level before maintenance injection) should be 15–35 μmol/hr/mL. This minimum trough level will ensure that plasma ADA activity from injection to injection is maintained above the level of total erythrocyte ADA activity in the blood of normal individuals.
Plasma ADA activity (pre-injection) should be determined every 1-2 weeks during the first 8-12 weeks of treatment in order to establish an effective dose of ADAGEN® (pegademase bovine) Injection. After 2 months of maintenance treatment with ADAGEN® (pegademase bovine) Injection, red cell dATP levels should decrease to a range of ≤ 0.005 to 0.015 μmol/mL. The normal value of dATP is below 0.001 μmol/mL. Once the level of dATP has fallen adequately, it should be measured 2-4 times a year during the remainder of the first year and 2-3 times a year thereafter, assuming no interruption in therapy.
Between 3 and 9 months, plasma ADA should be determined twice a month, then monthly until after 18-24 months of treatment with ADAGEN® (pegademase bovine) Injection.
Patients who have successfully been maintained on therapy for two years should continue to have plasma ADA measured every 2-4 months and red cell dATP measured twice yearly. More frequent monitoring would be necessary if therapy were interrupted or if an enhanced rate of clearance of plasma ADA activity develops.
Once effective ADA plasma levels have been established, should a patient's plasma ADA activity level fall below 10 μmol/hr/mL (which cannot be attributed to improper dosing, sample handling or antibody development) then the patients receiving this lot of ADAGEN® (pegademase bovine) Injection should be requested to have a blood sample for plasma ADA determination taken prior to their next injection of ADAGEN® (pegademase bovine) Injection.
Immune function, including the ability to produce antibodies, generally improves after 2-6 months of therapy, and matures over a longer period. Compared with the natural history of combined immunodeficiency disease due to ADA deficiency, a trend toward diminished frequency of opportunistic infections and fewer complications of infections has occurred in patients receiving ADAGEN® (pegademase bovine) Injection. However, the lag between the correction of the metabolic abnormalities and improved immune function with a trend toward diminished frequency of infections and complications of infection is variable, and has ranged from a few weeks to approximately 6 months. Improvement in the general clinical status of the patient may be gradual (as evidenced by improvement in various clinical parameters) but should be apparent by the end of the first year of therapy. Antibody to ADAGEN® (pegademase bovine) Injection may develop in patients and may result in more rapid clearance of ADAGEN® (pegademase bovine) Injection. Antibody to ADAGEN® (pegademase bovine) Injection should be suspected if a persistent fall in pre-injection levels of plasma ADA to < 10 μmol/hr/mL occurs. If other causes for a decline in plasma ADA levels can be ruled out [such as improper storage of ADAGEN® (pegademase bovine) Injection vials (freezing or prolonged storage at temperatures above 8°C), or improper handling of plasma samples (e.g., repeated freezing and thawing during transport to laboratory)], then a specific assay for antibody to ADA and ADAGEN® (pegademase bovine) Injection (ELISA, enzyme inhibition) should be performed.
In patients undergoing treatment with ADAGEN® (pegademase bovine) Injection, a decline in immune function, with increased risk of opportunistic infections and complications of infection, will result from failure to maintain adequate levels of plasma ADA activity [whether due to the development of antibody to ADAGEN® (pegademase bovine) Injection, to improper calculation of ADAGEN® (pegademase bovine) Injection dosage, to interruption of treatment or to improper storage of ADAGEN® (pegademase bovine) Injection with subsequent loss of activity]. If a persistent decline in plasma ADA activity occurs, immune function and clinical status should be monitored closely and precautions should be taken to minimize the risk of infection. If antibody to ADA or ADAGEN® (pegademase bovine) Injection is found to be the cause of a persistent fall in plasma ADA activity, then adjustment in the dosage of ADAGEN® (pegademase bovine) Injection and other measures may be taken to induce tolerance and restore adequate ADA activity.
Drug Interactions
There are no known drug interactions with ADAGEN® (pegademase bovine) Injection. However, Vidarabine is a substrate for ADA and 2′-deoxycoformycin is a potent inhibitor of ADA. Thus, the activities of these drugs and ADAGEN® (pegademase bovine) Injection could be substantially altered if they are used in combination with one another.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenic studies in animals have not been performed with ADAGEN® (pegademase bovine) Injection nor have studies been performed on impairment of fertility.
ADAGEN® (pegademase bovine) Injection did not exhibit a mutagenic effect when tested against Salmonella typhimurium strains in the Ames assay.
Pregnancy
Animal reproduction studies have not been conducted with ADAGEN® (pegademase bovine) Injection. It is also not known whether ADAGEN® (pegademase bovine) Injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. ADAGEN® (pegademase bovine) Injection should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS
Clinical experience with ADAGEN® (pegademase bovine) Injection has been limited. The following adverse reactions were reported: headache in one patient and pain at the injection site in two patients. The following adverse reactions have been identified during post-approval use of ADAGEN® (pegademase bovine) Injection. Because these reactions are reported voluntarily from a very small population, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hematologic events: hemolytic anemia, auto-immune hemolytic anemia, thrombocythemia, thrombocytopenia and autoimmune thrombocytopenia.
Dermatological events: injection site erythema, urticaria.
Lymphomas
To report SUSPECTED ADVERSE REACTIONS, contact Leadiant Biosciences, Inc. at 1-888-393-4584 or by email at drugsafety@leadiant.com or contact the FDA at 1-800-FDA-1088 or www.fda.gov/safety/medwatch.
OVERDOSAGE
There is no documented experience with ADAGEN® (pegademase bovine) Injection overdosage. An intraperitoneal dose of 50,000 U/kg of ADAGEN® (pegademase bovine) Injection in mice resulted in weight loss up to 9%.
DOSAGE AND ADMINISTRATION
Before prescribing ADAGEN® (pegademase bovine) Injection the physician should be thoroughly familiar with the details of this prescribing information. For further information concerning the essential monitoring of ADAGEN® (pegademase bovine) Injection therapy, the prescribing physician should contact Leadiant Biosciences, Inc., Gaithersburg, MD 20878. Telephone 1-866-792-5172.
ADAGEN® (pegademase bovine) Injection is recommended for use in infants from birth or in children of any age at the time of diagnosis.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permits.
ADAGEN® (pegademase bovine) Injection should not be diluted nor mixed with any other drug prior to administration.
ADAGEN® (pegademase bovine) Injection should be administered every 7 days as an intramuscular injection. The dosage of ADAGEN® (pegademase bovine) Injection should be individualized. The recommended dosing schedule is 10 U/kg for the first dose, 15 U/kg for the second dose, and 20 U/kg for the third dose. The usual maintenance dose is 20 U/kg per week. Further increases of 5 U/kg/week may be necessary, but a maximum single dose of 30 U/kg should not be exceeded. Plasma levels of ADA more than twice the upper limit of 35 μmol/hr/mL have occurred on occasion in several patients, and have been maintained for several weeks in one patient who received twice weekly injections (20 U/kg per dose) of ADAGEN® (pegademase bovine) Injection. No adverse effects have been observed at these higher levels; there is no evidence that maintaining pre-injection plasma ADA above 35 μmol/hr/mL produces any additional clinical benefits.
Dose proportionality has not been established and patients should be closely monitored when the dosage is increased. ADAGEN® (pegademase bovine) Injection is not recommended for intravenous administration.
The optimal dosage and schedule of administration should be established for each patient based on monitoring of plasma ADA activity levels (trough levels before maintenance injection) and biochemical markers of ADA deficiency (primarily red cell dATP content). Since improvement in immune function follows correction of metabolic abnormalities, maintenance dosage in individual patients should be aimed at achieving the following biochemical goals: 1) maintain plasma ADA activity (trough levels before maintenance injection) in the range of 15-35 μmol/hr/mL (assayed at 37°C); and 2) decline in erythrocyte dATP to ≤ 0.005-0.015 μmol/mL packed erythrocytes, or ≤ 1% of the total erythrocyte adenine nucleotide (ATP + dATP) content, with a normal ATP level, as measured in a pre-injection sample. In addition, continued monitoring of immune function and clinical status is essential in any patient with a primary immunodeficiency disease and should be continued in patients undergoing treatment with ADAGEN® (pegademase bovine) Injection.
HOW SUPPLIED
ADAGEN® (pegademase bovine) Injection is a clear, colorless, preservative free solution for intramuscular injection. ADAGEN® is supplied as a sterile solution in single-use vials containing 375 units per 1.5 mL solution, in boxes of 4 vials (NDC-57665-001-01).
Use only one dose per vial; do not re-enter the vial. Discard unused portions. Do not save unused drug for later administration.
Refrigerate. Store between +2°C and +8°C (36°F and 46°F). DO NOT FREEZE. ADAGEN® (pegademase bovine) Injection should not be stored at room temperature. This product should not be used if there are any indications that it may have been frozen.
Manufactured by Exelead, Inc., Indianapolis, IN 46268.
Distributed by Leadiant Biosciences, Inc., Gaithersburg, MD 20878.
REFERENCES
- Hershfield MS, Buckley RH, Greenberg ML, et al. Treatment of adenosine deaminase deficiency with polyethylene glycol-modified adenosine deaminase. N Engl J Med 1987; 316:589-96.
- Levy Y, Hershfield MS, Fernandez-Mejia C, Polmar ST, Scudiery D, Berger M, Sorensen RU. Adenosine deaminase deficiency with late onset of recurrent infections: response to treatment with polyethylene glycolmodified adenosine deaminase. J Pediatr 1988; 113:312-17.
- Kredich NM, Hershfield MS. Immunodeficiency diseases caused by adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency. 6th ed. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic basis of inherited disease. New York: McGraw Hill, 1989; 1045-75.
- Hirschhorn R. Inherited enzyme deficiencies and immunodeficiency: adenosine deaminase (ADA) and purine nucleoside phosphorylase (PNP) deficiencies. Clin Immunol Immunopathol 1986; 40:157-65.
- Hirschhorn R, Roegner-Maniscalco V, Kuritsky L, Rosen FS. Bone marrow transplantation only partially restores purine metabolites to normal adenosine deaminase-deficient patients. J Clin Invest 1981; 68:1387-93.
- Polmar AH, Stern RC, Schwartz AL, Wetzler EM, Chase PA, Hirschhorn R. Enzyme replacement therapy for adenosine deaminase deficiency and severe combined immunodeficiency. N Engl J Med 1976; 295:1337-43.
- Rubinstein A, Hirschhorn R, Sicklick M, Murphy RA. In vivo and in vitro effects of thymosin and adenosine deaminase on adenosine-deaminase-deficient lymphocytes. N Engl J Med 1979; 300:387-92.
- Hirschhorn R, Papageorgiou PS, Kesarwala HH, Taft LT. Amelioration of neurologic abnormalities after "enzyme replacement" in adenosine deaminase deficiency. N Engl J Med 1980; 303:377-80.
- Hirschhorn R, Ratech H, Rubinstein A, et al. Increased excretion of modified adenine nucleosides by children with adenosine deaminase deficiency. Pediatr Res 1982; 16:362-9.
- Polmar SH. Enzyme replacement and other biochemical approaches to the therapy of adenosine deaminase deficiency. In: Elliott K, Whelan J, eds. Enzyme defects and immune dysfunction. Amsterdam: Excerpta Medica, 1979; 213-30.
All Rights Reserved
Revised 11/2017
I-001-17-US-H
PRINCIPAL DISPLAY PANEL - CARTON LABEL
NDC: 57665-001-01
ADAGEN®
(pegademase bovine)
Injection
Four 1.5 mL single-dose vials.
250 units per mL
Sterile-For intramuscular use only.
See package insert for dosage information.
Discard if cloudy or frozen.
Store at + 2° C to + 8° C (36° F to 46° F)
REFRIGERATE-DO NOT FREEZE
Inactive Ingredients: 1.2 mg Monobasic
Sodium Phosphate, 5.58 mg Dibasic
Sodium Phosphate, 8.5 mg Sodium
Chloride, and Water for Injection
qs to 1.0 mL. Contains no preservative.
Rx Only
Leadiant
Biosciences
Distributed by: Leadiant
Biosciences, Inc.
Gaithersburg, MD 20878
Manufactured by: Exelead, Inc.
Indianapolis, IN 46268
| ADAGEN
pegademase bovine injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Leadiant Biosciences, Inc. (068301431) |