ROSUVASTATIN CALCIUM tablet
Rosuvastatin Calcium by
Drug Labeling and Warnings
Rosuvastatin Calcium by is a Prescription medication manufactured, distributed, or labeled by Umedica Laboratories USA Inc., UMEDICA LABORATORIES PRIVATE LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ROSUVASTATIN TABLETS, safely and effectively. See full prescribing information for ROSUVASTATIN TABLETS.
ROSUVASTATIN tablets, for oral use
Initial U.S. Approval: 2003RECENT MAJOR CHANGES
Indications and Usage ( 1) 07/2024
INDICATIONS AND USAGE
Rosuvastatin tablet is an HMG Co-A reductase inhibitor (statin) indicated: ( 1)
- To reduce the risk of major adverse cardiovascular (CV) events (CV death, nonfatal myocardial infarction, nonfatal stroke, or an arterial revascularization procedure) in adults without established coronary heart disease who are at increased risk of CV disease based on age, high-sensitivity C-reactive protein (hsCRP) ≥2 mg/L, and at least one additional CV risk factor.
- As an adjunct to diet to:
- reduce LDL-C in adults with primary hyperlipidemia.
- reduce LDL-C and slow the progression of atherosclerosis in adults.
- reduce LDL-C in adults and pediatric patients aged 8 years and older with heterozygous familial hypercholesterolemia (HeFH).
- As an adjunct to other LDL-C-lowering therapies, or alone if such treatments are unavailable, to reduce LDL-C in adults and pediatric patients aged 7 years and older with homozygous familial hypercholesterolemia (HoFH).
- As an adjunct to diet for the treatment of adults with:
- Primary dysbetalipoproteinemia.
- Hypertriglyceridemia.
DOSAGE AND ADMINISTRATION
Take orally with or without food, at any time of day. ( 2.1)
Assess LDL-C when clinically appropriate, as early as 4 weeks after initiating rosuvastatin tablets, and adjust dosage if necessary. ( 2.1)
Adults:Recommended dosage range is 5 to 40 mg once daily. ( 2.1)
Pediatric Patients with HeFH: Recommended dosage range is 5 to 10 mg once daily for patients aged 8 to less than 10 years of age, and 5 to 20 mg once daily for patients aged 10 years and older. ( 2.2)
Pediatric Patients with HoFH: Recommended dosage is 20 mg once daily for patients aged 7 years and older. ( 2.2)
Asian Patients: Initiate at 5 mg once daily. Consider risks and benefits of treatment if not adequately controlled at doses up to 20 mg once daily. ( 2.4)
Patients with Severe Renal Impairment (not on hemodialysis): Initiate at 5 mg once daily; do not exceed 10 mg once daily. ( 2.5)
See full prescribing information for rosuvastatin tablet dosage and administration modifications due to drug interactions. ( 2.6)
DOSAGE FORMS AND STRENGTHS
Tablets: 5 mg, 10 mg, 20 mg, and 40 mg of rosuvastatin. ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Myopathy and Rhabdomyolysis: Risk factors include age 65 years or greater, uncontrolled hypothyroidism, renal impairment, concomitant use with certain other drugs, and higher rosuvastatin tablets dosage. Asian patients may be at higher risk for myopathy. Discontinue rosuvastatin tablets if markedly elevated CK levels occur or myopathy is diagnosed or suspected. Temporarily discontinue rosuvastatin tablets in patients experiencing an acute or serious condition at high risk of developing renal failure secondary to rhabdomyolysis. Inform patients of the risk of myopathy and rhabdomyolysis when starting or increasing rosuvastatin tablets dosage. Instruct patients to promptly report unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever. ( 5.1)
- Immune-Mediated Necrotizing Myopathy (IMNM): Rare reports of IMNM, an autoimmune myopathy, have been reported with statin use. Discontinue rosuvastatin tablets if IMNM is suspected. ( 5.2)
- Hepatic Dysfunction: Increases in serum transaminases have occurred, some persistent. Rare reports of fatal and non-fatal hepatic failure have occurred. Consider testing liver enzymes before initiating therapy and as clinically indicated thereafter. If serious hepatic injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs, promptly discontinue rosuvastatin tablets. ( 5.3)
ADVERSE REACTIONS
Most frequent adverse reactions (rate ≥2%) are headache, nausea, myalgia, asthenia, and constipation. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Umedica Laboratories USA Inc. at 1-855-288-5777 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
See full prescribing information for details regarding concomitant use of rosuvastatin tablets with other drugs that increase the risk of myopathy and rhabdomyolysis. ( 7.1)
Aluminum and Magnesium Hydroxide Combination Antacids:Administer rosuvastatin tablets at least 2 hours before the antacid. ( 7.2)
Warfarin:Obtain INR prior to starting rosuvastatin tablets. Monitor INR frequently until stable upon initiation, dose titration or discontinuation. ( 7.3)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosage and Administration Information
2.2 Recommended Dosage in Adult Patients
2.3 Recommended Dosage in Pediatric Patients
2.4 Dosing in Asian Patients
2.5 Recommended Dosage in Patients with Renal Impairment
2.6 Dosage Modifications Due to Drug Interactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myopathy and Rhabdomyolysis
5.2 Immune-Mediated Necrotizing Myopathy
5.3 Hepatic Dysfunction
5.4 Proteinuria and Hematuria
5.5 Increases in HbA1c and Fasting Serum Glucose Levels
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drug Interactions that Increase the Risk of Myopathy and Rhabdomyolysis with Rosuvastatin Tablets
7.2 Drug Interactions that Decrease the Efficacy of Rosuvastatin Tablets
7.3 Rosuvastatin Tablets Effects on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Asian Patients
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Rosuvastatin tablets is indicated:
- To reduce the risk of major adverse cardiovascular (CV) events (CV death, nonfatal myocardial infarction, nonfatal stroke, or an arterial revascularization procedure) in adults without established coronary heart disease who are at increased risk of CV disease based on age, high-sensitivity C-reactive protein (hsCRP) ≥2 mg/L, and at least one additional CV risk factor.
- As an adjunct to diet to:
- Reduce low-density lipoprotein cholesterol (LDL-C) in adults with primary hyperlipidemia.
- Reduce LDL-C and slow the progression of atherosclerosis in adults.
- Reduce LDL-C in adults and pediatric patients aged 8 years and older with heterozygous familial hypercholesterolemia (HeFH).
- As an adjunct to other LDL-C-lowering therapies, or alone if such treatments are unavailable, to reduce LDL-C in adults and pediatric patients aged 7 years and older with homozygous familial hypercholesterolemia (HoFH).
- As an adjunct to diet for the treatment of adults with:
- Primary dysbetalipoproteinemia.
- Hypertriglyceridemia.
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosage and Administration Information
- Administer rosuvastatin tablets orally as a single dose at any time of day, with or without food. Swallow the tablets whole.
- Assess LDL-C when clinically appropriate, as early as 4 weeks after initiating, rosuvastatin tablets and adjust the dosage if necessary.
- If a dose is missed, advise patients not to take an extra dose. Resume treatment with the next dose.
- When taking rosuvastatin tablets with an aluminum and magnesium hydroxide combination antacid, administer rosuvastatin tablets at least 2 hours before the antacid [see Drug Interactions (7.2)].
2.2 Recommended Dosage in Adult Patients
- The dosage range for rosuvastatin tablets is 5 to 40 mg orally once daily.
- The recommended dose of rosuvastatin tablets depends on a patient’s indication for usage, LDL-C, and individual risk for CV events.
2.3 Recommended Dosage in Pediatric Patients
Dosage in Pediatric Patients 8 Years of Age and Older with HeFH
The recommended dosage range is 5 mg to 10 mg orally once daily in patients aged 8 years to less than 10 years and 5 mg to 20 mg orally once daily in patients aged 10 years and older.Dosage in Pediatric Patients 7 Years of Age and Older with HoFH
The recommended dosage is 20 mg orally once daily.2.4 Dosing in Asian Patients
Initiate rosuvastatin tablets at 5 mg once daily due to increased rosuvastatin plasma concentrations. Consider the risks and benefits of rosuvastatin tablets when treating Asian patients not adequately controlled at doses up to 20 mg once daily [see Warnings and Precautions (5.1), Use in Specific Populations (8.8), and Clinical Pharmacology (12.3)] .
2.5 Recommended Dosage in Patients with Renal Impairment
In patients with severe renal impairment (CL crless than 30 mL/min/1.73 m 2) not on hemodialysis, the recommended starting dosage is 5 mg once daily and should not exceed 10 mg once daily [see Warnings and Precautions (5.1)and Use in Specific Populations (8.6)] .
There are no dosage adjustment recommendations for patients with mild and moderate renal impairment.
2.6 Dosage Modifications Due to Drug Interactions
Table 1 displays dosage modifications for Rosuvastatin tablets due to drug interactions [see Warnings and Precautions (5.1)and Drug Interactions (7.1)] .
Table 1: Rosuvastatin Tablets Dosage Modifications Due to Drug Interactions
Concomitantly Used Drug
Rosuvastatin tablets Dosage Modifications
Cyclosporine
Do not exceed 5 mg once daily.
Teriflunomide
Do not exceed 10 mg once daily.
Enasidenib
Do not exceed 10 mg once daily.
Capmatinib
Do not exceed 10 mg once daily.
Fostamatinib
Do not exceed 20 mg once daily.
Febuxostat
Do not exceed 20 mg once daily.
Gemfibrozil
Avoid concomitant use. If used concomitantly, initiate at 5 mg once daily and do not exceed 10 mg once daily.
Tafamidis
Avoid concomitant use. If used concomitantly, initiate at 5 mg once daily and do not exceed 20 mg once daily.
Antiviral Medications
- Sofbuvir/velpatasvir/voxilaprevir
- Ledipasvir/sofosbuvir
Concomitant use not recommended.
- Simeprevir
- Dasabuvir/ombitasvir/paritaprevir/ritonavir
- Elbasvir/Grazoprevir
- Sofosbuvir/Velpatasvir
- Glecaprevir/Pibrentasvir
- Atazanavir/Ritonavir
- Lopinavir/Ritonavir
Initiate at 5 mg once daily. Do not exceed 10 mg once daily.
Darolutamide
Do not exceed 5 mg once daily.
Regorafenib
Do not exceed 10 mg once daily.
-
3 DOSAGE FORMS AND STRENGTHS
- 5 mg: Pink, round, biconvex, beveled edge, film coated tablets debossed with “R5” on one side and plain on other side.
- 10 mg: Pink, round, biconvex, beveled edge, film coated tablets debossed with “R10” on one side and plain on other side.
- 20 mg: Pink, round, biconvex, film coated tablets debossed with “R20” on one side and plain on other side.
- 40 mg: Pink, oval, biconvex, film-coated tablets debossed with “R40” on one side and plain on other side.
-
4 CONTRAINDICATIONS
Rosuvastatin tablets is contraindicated in the following conditions:
- Acute liver failure or decompensated cirrhosis [see Warnings and Precautions (5.3)].
- Hypersensitivity to rosuvastatin or any excipients in rosuvastatin tablets. Hypersensitivity reactions including rash, pruritus, urticaria, and angioedema have been reported with rosuvastatin tablets [see Adverse Reactions (6.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Myopathy and Rhabdomyolysis
Rosuvastatin tablets may cause myopathy [muscle pain, tenderness, or weakness associated with elevated creatine kinase (CK)] and rhabdomyolysis. Acute kidney injury secondary to myoglobinuria and rare fatalities have occurred as a result of rhabdomyolysis with statins, including rosuvastatin tablets.
Risk Factors for Myopathy
Risk factors for myopathy include age 65 years or greater, uncontrolled hypothyroidism, renal impairment, concomitant use with certain other drugs (including other lipid-lowering therapies), and higher rosuvastatin tablets dosage. Asian patients on rosuvastatin tablets may be at higher risk for myopathy [see Drug Interactions (7.1)and Use in Specific Populations (8.8)]. The myopathy risk is greater in patients taking rosuvastatin tablets 40 mg daily compared with lower rosuvastatin tablets dosages.Steps to Prevent or Reduce the Risk of Myopathy and Rhabdomyolysis
The concomitant use of rosuvastatin tablets with cyclosporine or gemfibrozil is not recommended. rosuvastatin tablets dosage modifications are recommended for patients taking certain antiviral medications, darolutamide, and regorafenib [see Dosage and Administration (2.6)]. Niacin, fibrates, and colchicine may also increase the risk of myopathy and rhabdomyolysis [see Drug Interactions (7.1)].Discontinue rosuvastatin tablets if markedly elevated CK levels occur or if myopathy is either diagnosed or suspected. Muscle symptoms and CK elevations may resolve if rosuvastatin tablets is discontinued. Temporarily discontinue rosuvastatin tablets in patients experiencing an acute or serious condition at high risk of developing renal failure secondary to rhabdomyolysis (e.g., sepsis; shock; severe hypovolemia; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy).
Inform patients of the risk of myopathy and rhabdomyolysis when starting or increasing the rosuvastatin tablets dosage. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever.
5.2 Immune-Mediated Necrotizing Myopathy
There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use, including reports of recurrence when the same or a different statin was administered. IMNM is characterized by proximal muscle weakness and elevated serum creatine kinase that persist despite discontinuation of statin treatment; positive anti-HMG CoA reductase antibody; muscle biopsy showing necrotizing myopathy; and improvement with immunosuppressive agents. Additional neuromuscular and serologic testing may be necessary. Treatment with immunosuppressive agents may be required. Discontinue rosuvastatin tablets if IMNM is suspected.
5.3 Hepatic Dysfunction
Increases in serum transaminases have been reported with use of rosuvastatin tablets [see Adverse Reactions (6.1)]. In most cases, these changes appeared soon after initiation, were transient, were not accompanied by symptoms, and resolved or improved on continued therapy or after a brief interruption in therapy. In a pooled analysis of placebo-controlled trials, increases in serum transaminases to more than three times the ULN occurred in 1.1% of patients taking rosuvastatin tablets versus 0.5% of patients treated with placebo. Marked persistent increases of hepatic transaminases have also occurred with rosuvastatin tablets. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including rosuvastatin tablets.
Patients who consume substantial quantities of alcohol and/or have a history of liver disease may be at increased risk for hepatic injury [see Use in Specific Populations (8.7)].
Consider liver enzyme testing before rosuvastatin tablets initiation and when clinically indicated thereafter. Rosuvastatin tablets is contraindicated in patients with acute liver failure or decompensated cirrhosis [see Contraindications (4)]. If serious hepatic injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs, promptly discontinue rosuvastatin tablets.
5.4 Proteinuria and Hematuria
In the Rosuvastatin tablets clinical trial program, dipstick-positive proteinuria and microscopic hematuria were observed among rosuvastatin tablets treated patients. These findings were more frequent in patients taking rosuvastatin tablets 40 mg, when compared to lower doses of rosuvastatin tablets or comparator statins, though it was generally transient and was not associated with worsening renal function. Although the clinical significance of this finding is unknown, consider a dose reduction for patients on rosuvastatin tablets therapy with unexplained persistent proteinuria and/or hematuria during routine urinalysis testing.
5.5 Increases in HbA1c and Fasting Serum Glucose Levels
Increases in HbA1c and fasting serum glucose levels have been reported with statins, including rosuvastatin tablets. Based on clinical trial data with rosuvastatin tablets, in some instances these increases may exceed the threshold for the diagnosis of diabetes mellitus [see Adverse Reactions (6.1)] . Optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices.
-
6 ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
Myopathy and Rhabdomyolysis [see Warnings and Precautions (5.1)]
Immune-Mediated Necrotizing Myopathy [see Warnings and Precautions (5.2)]
Hepatic Dysfunction [see Warnings and Precautions (5.3)]
Proteinuria and Hematuria [see Warnings and Precautions (5.4)]
Increases in HbA1c and Fasting Serum Glucose Levels [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trails are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trails of another drug and may not reflect the rates observed in clinical practice.
Adverse reactions reported in ≥2% of patients in placebo-controlled clinical studies and at a rate greater than placebo are shown in Table 2. These studies had a treatment duration of up to 12 weeks.
Table 2: Adverse Reactions Reported in ≥2% of Patients Treated with Rosuvastatin Tablets and > Placebo in Placebo-Controlled Trials
Adverse Reactions
Placebo
N=382
%Rosuvastatin
5 mg
N=291
%Rosuvastatin
10 mg
N=283
%Rosuvastatin
20 mg
N=64
%Rosuvastatin
40 mg
N=106
%Total
Rosuvastatin
5 mg-40 mg
N=744
%Headache
5.0
5.5
4.9
3.1
8.5
5.5
Nausea
3.1
3.8
3.5
6.3
0
3.4
Myalgia
1.3
3.1
2.1
6.3
1.9
2.8
Asthenia
2.6
2.4
3.2
4.7
0.9
2.7
Constipation
2.4
2.1
2.1
4.7
2.8
2.4
Other adverse reactions reported in clinical studies were abdominal pain, dizziness, hypersensitivity (including rash, pruritus, urticaria, and angioedema) and pancreatitis. The following laboratory abnormalities have also been reported: dipstick-positive proteinuria and microscopic hematuria; elevated creatine phosphokinase, transaminases, glucose, glutamyl transpeptidase, alkaline phosphatase, and bilirubin; and thyroid function abnormalities.
In the METEOR study, patients were treated with rosuvastatin tablets 40 mg (n=700) or placebo (n=281) with a mean treatment duration of 1.7 years. Adverse reactions reported in ≥2% of patients and at a rate greater than placebo are shown in Table 3.
Table 3: Adverse Reactions Reported in ≥2% of Patients Treated with Rosuvastatin Tablets and > Placebo in the METEOR Trial
Adverse Reactions
Placebo
N=281
%Rosuvastatin Tablets 40 mg
N=700
%Myalgia
12.1
12.7
Arthralgia
7.1
10.1
Headache
5.3
6.4
Dizziness
2.8
4.0
Increased CPK
0.7
2.6
Abdominal pain
1.8
2.4
ALT greater than 3x ULN 1
0.7
2.2
1Frequency recorded as abnormal laboratory value.
In the JUPITER study, patients were treated with rosuvastatin tablets 20 mg (n=8,901) or placebo (n=8,901) for a mean duration of 2 years. In JUPITER, there was a significantly higher frequency of diabetes mellitus reported in patients taking rosuvastatin tablets (2.8%) versus patients taking placebo (2.3%). Mean HbA1c was significantly increased by 0.1% in rosuvastatin tablets-treated patients compared to placebo-treated patients. The number of patients with a HbA1c >6.5% at the end of the trial was significantly higher in rosuvastatin tablets-treated versus placebo-treated patients [see Clinical Studies (14)].
Adverse reactions reported in ≥2% of patients and at a rate greater than placebo are shown in Table 4.
Table 4: Adverse Reactions Reported in ≥2% of Patients Treated with Rosuvastatin Tablets and > Placebo in the JUPITER Trial
Adverse Reactions
Placebo
N=8,901 %
Rosuvastatin Tablets 20 mg
N=8,901 %
Myalgia
6.6
7.6
Arthralgia
3.2
3.8
Constipation
3.0
3.3
Diabetes mellitus
2.3
2.8
Nausea
2.3
2.4
Pediatric Patients with HeFH
In a 12-week controlled study in pediatric patients 10 to 17 years of age with HeFH with rosuvastatin tablets 5 mg to 20 mg daily [see Use in Specific Populations (8.4)and Clinical Studies (14)], elevations in serum CK greater than 10 x ULN were observed more frequently in rosuvastatin tablets treated patients compared with patients receiving placebo. Four of 130 (3%) patients treated with rosuvastatin tablets (2 treated with 10 mg and 2 treated with 20 mg) had increased CK greater than 10 x ULN, compared to 0 of 46 patients on placebo.6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of rosuvastatin tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood Disorders: thrombocytopenia
Hepatobiliary Disorders: hepatitis, jaundice, fatal and non-fatal hepatic failure
Musculoskeletal Disorders: arthralgia, rare reports of immune-mediated necrotizing myopathy associated with statin use
Nervous System Disorders: peripheral neuropathy, rare postmarketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, and confusion) associated with the use of all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).There have been rare reports of new-onset or exacerbation of myasthenia gravis, including ocular myasthenia, and reports of recurrence when the same or a different statin was administered.
Psychiatric Disorders: depression, sleep disorders (including insomnia and nightmares)
Reproductive System and Breast Disorders: gynecomastia
Respiratory Disorders: interstitial lung disease
Skin and Subcutaneous Tissue Disorders: drug reaction with eosinophilia and systemic symptoms (DRESS), lichenoid drug eruption -
7 DRUG INTERACTIONS
7.1 Drug Interactions that Increase the Risk of Myopathy and Rhabdomyolysis with Rosuvastatin Tablets
Rosuvastatin is a substrate of CYP2C9 and transporters (such as OATP1B1, BCRP). Rosuvastatin plasma levels can be significantly increased with concomitant administration of inhibitors of CYP2C9 and transporters. Table 5 includes a list of drugs that increase the risk of myopathy and rhabdomyolysis when used concomitantly with rosuvastatin tablets and instructions for preventing or managing them [see Warnings and Precautions (5.1)and Clinical Pharmacology (12.3)].
Table 5: Drug Interactions that Increase the Risk of Myopathy and Rhabdomyolysis with Rosuvastatin Tablets
Cyclosporine
Clinical Impact:
Cyclosporine increased rosuvastatin exposure 7-fold. The risk of myopathy and rhabdomyolysis is increased with concomitant use of cyclosporine or gemfibrozil with rosuvastatin tablets.
Intervention:
If used concomitantly, do not exceed a dose of rosuvastatin tablets 5 mg once daily.
Teriflunomide
Clinical Impact:
Teriflunomide increased rosuvastatin exposure more than 2.5-fold. The risk of myopathy and rhabdomyolysis is increased with concomitant use.
Intervention:
In patients taking teriflunomide, do not exceed a dose of rosuvastatin tablets 10 mg once daily.
Enasidenib
Clinical Impact:
Enasidenib increased rosuvastatin exposure more than 2.4-fold. The risk of myopathy and rhabdomyolysis is increased with concomitant use.
Intervention:
In patients taking enasidenib, do not exceed a dose of rosuvastatin tablets 10 mg once daily.
Capmatinib
Clinical Impact:
Capmatinib increased rosuvastatin exposure more than 2.1-fold. The risk of myopathy and rhabdomyolysis is increased with concomitant use.
Intervention:
In patients taking capmatinib, do not exceed a dose of rosuvastatin tablets 10 mg once daily.
Fostamatinib
Clinical Impact:
Fostamatinib increased rosuvastatin exposure more than 2.0-fold. The risk of myopathy and rhabdomyolysis is increased with concomitant use.
Intervention:
In patients taking fostamatinib, do not exceed a dose of rosuvastatin tablets 20 mg once daily.
Febuxostat
Clinical Impact:
Febuxostat increased rosuvastatin exposure more than 1.9-fold. The risk of myopathy and rhabdomyolysis is increased with concomitant use.
Intervention:
In patients taking febuxostat, do not exceed a dose of rosuvastatin tablets 20 mg once daily.
Gemfibrozil
Clinical Impact:
Gemfibrozil significantly increased rosuvastatin exposure and gemfibrozil may cause myopathy when given alone. The risk of myopathy and rhabdomyolysis is increased with concomitant use of gemfibrozil with rosuvastatin tablets.
Intervention:
Avoid concomitant use of gemfibrozil with rosuvastatin tablets. If used concomitantly, initiate rosuvastatin tablets at 5 mg once daily and do not exceed a dose of rosuvastatin tablets 10 mg once daily.
Tafamidis
Clinical Impact:
Tafamidis significantly increased rosuvastatin exposure and tafamidis may cause myopathy when given alone. The risk of myopathy and rhabdomyolysis is increased with concomitant use of tafamidis with rosuvastatin tablets.
Intervention:
Avoid concomitant use of tafamidis with rosuvastatin tablets. If used concomitantly, initiate rosuvastatin tablets at 5 mg once daily and do not exceed a dose of rosuvastatin tablets 20 mg once daily. Monitor for signs of myopathy and rhabdomyolysis if used concomitantly with rosuvastatin tablets.
Anti-Viral Medications
Clinical Impact:
Rosuvastatin plasma levels were significantly increased with concomitant administration of many anti-viral drugs, which increases the risk of myopathy and rhabdomyolysis.
Intervention:
Sofosbuvir/velpatasvir/voxilaprevir
Ledipasvir/sofosbuvirAvoid concomitant use with rosuvastatin tablets.
Simeprevir
Dasabuvir/ombitasvir/paritaprevir/ritonavir
Elbasvir/grazoprevir
Sofosbuvir/velpatasvir
Glecaprevir/pibrentasvir
Atazanavir/ritonavir
Lopinavir/ritonavirInitiate with rosuvastatin tablets 5 mg once daily, and do not exceed a dose of rosuvastatin tablets 10 mg once daily.
Darolutamide
Clinical Impact:
Darolutamide increased rosuvastatin exposure more than 5-fold. The risk of myopathy and rhabdomyolysis is increased with concomitant use.
Intervention:
In patients taking darolutamide, do not exceed a dose of rosuvastatin tablets 5 mg once daily.
Regorafenib
Clinical Impact:
Regorafenib increased rosuvastatin exposure and may increase the risk of myopathy.
Intervention:
In patients taking regorafenib, do not exceed a dose of rosuvastatin tablets 10 mg once daily.
Fenofibrates (e.g., fenofibrate and fenofibric acid)
Clinical Impact:
Fibrates may cause myopathy when given alone. The risk of myopathy and rhabdomyolysis is increased with concomitant use of fibrates with rosuvastatin tablets.
Intervention:
Consider if the benefit of using fibrates concomitantly with rosuvastatin tablets outweighs the increased risk of myopathy and rhabdomyolysis. If concomitant use is decided, monitor patients for signs and symptoms of myopathy, particularly during initiation of therapy and during upward dose titration of either drug.
Niacin
Clinical Impact:
Cases of myopathy and rhabdomyolysis have occurred with concomitant use of lipid-modifying doses (≥1 g/day) of niacin with rosuvastatin tablets.
Intervention:
Consider if the benefit of using lipid-modifying doses (≥1 g/day) of niacin concomitantly with rosuvastatin tablets outweighs the increased risk of myopathy and rhabdomyolysis. If concomitant use is decided, monitor patients for signs and symptoms of myopathy, particularly during initiation of therapy and during upward dose titration of either drug.
Colchicine
Clinical Impact:
Cases of myopathy and rhabdomyolysis have been reported with concomitant use of colchicine with rosuvastatin tablets.
Intervention:
Consider if the benefit of using colchicine concomitantly with rosuvastatin tablets outweighs the increased risk of myopathy and rhabdomyolysis. If concomitant use is decided, monitor patients for signs and symptoms of myopathy, particularly during initiation of therapy and during upward dose titration of either drug.
Ticagrelor
Clinical Impact:
Concomitant use of rosuvastatin tablets and ticagrelor has been shown to increase rosuvastatin concentrations, which may result in increased risk of myopathy. Cases of myopathy and rhabdomyolysis have been reported in patients using both products concomitantly. Cases have occurred more frequently in patients taking 40 mg of rosuvastatin.
Intervention:
In patients taking concomitant ticagrelor, especially those with additional risk factors for myopathy and rhabdomyolysis, monitor patients for signs and symptoms of myopathy, particularly during initiation of therapy and during upward dose titration of rosuvastatin tablets.
7.2 Drug Interactions that Decrease the Efficacy of Rosuvastatin Tablets
Table 6 presents drug interactions that may decrease the efficacy of rosuvastatin tablets and instructions for preventing or managing them.
Table 6: Drug Interactions that Decrease the Efficacy of Rosuvastatin Tablets
Antacids
Clinical Impact:
Concomitant aluminum and magnesium hydroxide combination antacid administration decreased the mean exposure of rosuvastatin 50% [see Clinical Pharmacology (12.3)] .
Intervention:
In patients taking antacid, administer rosuvastatin tablets at least 2 hours before the antacid .
7.3 Rosuvastatin Tablets Effects on Other Drugs
Table 7 presents rosuvastatin tablets’s effect on other drugs and instructions for preventing or managing them.
Table 7: Rosuvastatin Tablets Effects on Other Drugs
Warfarin
Clinical Impact:
Rosuvastatin significantly increased the INR in patients receiving warfarin [see Clinical Pharmacology (12.3)] .
Intervention:
In patients taking warfarin, obtain an INR before starting rosuvastatin tablets and frequently enough after initiation, dose titration or discontinuation to ensure that no significant alteration in INR occurs. Once the INR is stable, monitor INR at regularly recommended intervals.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Discontinue rosuvastatin tablets when pregnancy is recognized. Alternatively, consider the ongoing therapeutic needs of the individual patient.Rosuvastatin tablets decreases synthesis of cholesterol and possibly other biologically active substances derived from cholesterol; therefore, rosuvastatin tablets may cause fetal harm when administered to pregnant patients based on the mechanism of action [see Clinical Pharmacology (12.1)]. In addition, treatment of hyperlipidemia is not generally necessary during pregnancy. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hyperlipidemia for most patients.
Available data from case series and prospective and retrospective observational cohort studies over decades of use with statins in pregnant women have not identified a drug-associated risk of major congenital malformations. Published data from prospective and retrospective observational cohort studies with rosuvastatin tablets use in pregnant women are insufficient to determine if there is a drug-associated risk of miscarriage (see Data).
In animal reproduction studies, no adverse developmental effects were observed in pregnant rats or rabbits orally administered rosuvastatin during the period of organogenesis at doses that resulted in systemic exposures equivalent to human exposures at the maximum recommended human dose (MRHD) of 40 mg/day, based on AUC and body surface area (mg/m 2), respectively (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
A Medicaid cohort linkage study of 1,152 statin-exposed pregnant women compared to 886,996 controls did not find a significant teratogenic effect from maternal use of statins in the first trimester of pregnancy, after adjusting for potential confounders – including maternal age, diabetes mellitus, hypertension, obesity, and alcohol and tobacco use – using propensity score-based methods. The relative risk of congenital malformations between the group with statin use and the group with no statin use in the first trimester was 1.07 (95% confidence interval 0.85 to 1.37) after controlling for confounders, particularly pre-existing diabetes mellitus. There were also no statistically significant increases in any of the organ-specific malformations assessed after accounting for confounders. In the majority of pregnancies, statin treatment was initiated prior to pregnancy and was discontinued at some point in the first trimester when pregnancy was identified. Study limitations include reliance on physician coding to define the presence of a malformation, lack of control for certain confounders such as body mass index, use of prescription dispensing as verification for the use of a statin, and lack of information on non-live births.Animal Data
In female rats given 5, 15 and 50 mg/kg/day before mating and continuing through to gestation day 7 resulted in decreased fetal body weight (female pups) and delayed ossification at 50 mg/kg/day (10 times the human exposure at the MRHD dose of 40 mg/day based on AUC).In pregnant rats given 2, 10 and 50 mg/kg/day of rosuvastatin from gestation day 7 through lactation day 21 (weaning), decreased pup survival occurred at 50 mg/kg/day (dose equivalent to 12 times the MRHD of 40 mg/day based body surface area).
In pregnant rabbits given 0.3, 1, and 3 mg/kg/day of rosuvastatin from gestation day 6 to day 18, decreased fetal viability and maternal mortality was observed at 3 mg/kg/day (dose equivalent to the MRHD of 40 mg/day based on body surface area).
Rosuvastatin crosses the placenta in rats and rabbits and is found in fetal tissue and amniotic fluid at 3% and 20%, respectively, of the maternal plasma concentration following a single 25 mg/kg oral gavage dose on gestation day 16 in rats. In rabbits, fetal tissue distribution was 25% of maternal plasma concentration after a single oral gavage dose of 1 mg/kg on gestation day 18.
8.2 Lactation
Risk Summary
Limited data from case reports in published literature indicate that rosuvastatin is present in human milk. There is no available information on the effects of the drug on the breastfed infant or the effects of the drug on milk production. Statins, including rosuvastatin, decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol and may cause harm to the breastfed infant.
Because of the potential for serious adverse reactions in a breastfed infant, based on the mechanism of action, advise patients that breastfeeding is not recommended during treatment with rosuvastatin tablets [see Use in Specific Populations (8.1)and Clinical Pharmacology (12.1)].
8.4 Pediatric Use
The safety and effectiveness of rosuvastatin tablets as an adjunct to diet to reduce LDL-C have been established in pediatric patients 8 years of age and older with HeFH. Use of rosuvastatin tablets for this indication is based on one 12-week controlled trial with a 40-week open-label extension period in 176 pediatric patients 10 years of age and older with HeFH and one 2-year open-label, uncontrolled trial in 175 pediatric patients 8 years of age and older with HeFH [see Clinical Studies (14)]. In the 1-year trial with a 12-week controlled phase, there was no detectable effect of rosuvastatin tablets on growth, weight, BMI (body mass index), or sexual maturation in patients aged 10 to 17 years.
The safety and effectiveness of rosuvastatin tablets as an adjunct to other LDL-C-lowering therapies to reduce LDL-C have been established in pediatric patients 7 years of age and older with HoFH. Use of rosuvastatin tablets for this indication is based on a randomized, placebo-controlled, cross-over study in 14 pediatric patients 7 years of age and older with HoFH [see Clinical Studies (14)].
The safety and effectiveness of rosuvastatin tablets have not been established in pediatric patients younger than 8 years of age with HeFH, younger than 7 years of age with HoFH, or in pediatric patients with other types of hyperlipidemia (other than HeFH or HoFH).
8.5 Geriatric Use
Of the 10,275 patients in clinical studies with rosuvastatin tablets, 3,159 (31%) were 65 years and older, and 698 (6.8%) were 75 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
Advanced age (≥65 years) is a risk factor for rosuvastatin tablets-associated myopathy and rhabdomyolysis. Dose selection for an elderly patient should be cautious, recognizing the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy and the higher risk of myopathy. Monitor geriatric patients receiving rosuvastatin tablets for the increased risk of myopathy [see Warnings and Precautions (5.1)] .
8.6 Renal Impairment
Rosuvastatin exposure is not influenced by mild to moderate renal impairment (CL cr≥30 mL/min/1.73 m 2). Exposure to rosuvastatin is increased to a clinically significant extent in patients with severe renal impairment (CL cr<30 mL/min/1.73 m 2) who are not receiving hemodialysis [see Clinical Pharmacology (12.3)].
Renal impairment is a risk factor for myopathy and rhabdomyolysis. Monitor all patients with renal impairment for development of myopathy. In patients with severe renal impairment not on hemodialysis, the recommended starting dosage is 5 mg daily and should not exceed 10 mg daily [see Dosage and Administration (2.5)and Warnings and Precautions (5.1)].
8.7 Hepatic Impairment
Rosuvastatin is contraindicated in patients with active liver failure or decompensated cirrhosis. Chronic alcohol liver disease is known to increase rosuvastatin exposure. Patients who consume substantial quantities of alcohol and/or have a history of liver disease may be at increased risk for hepatic injury [see Contraindications (4), Warning and Precautions (5.3)and Clinical Pharmacology (12.3)].
8.8 Asian Patients
Pharmacokinetic studies have demonstrated an approximate 2-fold increase in median exposure to rosuvastatin in Asian subjects when compared with White controls. Adjust the rosuvastatin tablets dosage in Asian patients [see Dosage and Administration (2.4)and Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Rosuvastatin calcium USP is a 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA)-reductase inhibitor.
The chemical name for rosuvastatin calcium USP is 6-Heptenoic acid, 7-[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl) amino]-5-pyrimidinyl]-3,5-dihydroxy-, calcium salt (2:1), (3R,5S,6E) with the following structural formula:
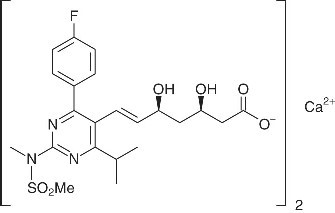
The empirical formula for rosuvastatin calcium USP is (C 22H 27FN 3O 6S) 2Ca and the molecular weight is 1,001.14. Rosuvastatin calcium USP is a White or almost white, hygroscopic powder that is freely soluble in methylene chloride, slightly soluble in water practically insoluble in anhydrous ethanol. Rosuvastatin calcium is a hydrophilic compound with a partition coefficient (octanol/water) of 0.13 at pH of 7.0.
Rosuvastatin tablets for oral use contain rosuvastatin 5 mg, 10 mg, 20 mg, or 40 mg (equivalent to 5.2 mg, 10.4 mg, 20.8 mg, and 41.6 mg rosuvastatin calcium) and the following inactive ingredients: Each tablet contains: crospovidone USP, hypromellose USP, iron oxide red NF, lactose monohydrate USP, magnesium stearate USP, microcrystalline cellulose USP, sodium bicarbonate powder NF, titanium dioxide USP, triacetin USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Rosuvastatin tablets is an inhibitor of HMG-CoA reductase, the rate-limiting enzyme that converts 3-hydroxy-3-methylglutaryl coenzyme A to mevalonate, a precursor of cholesterol.
12.2 Pharmacodynamics
Inhibition of HMG-CoA reductase by rosuvastatin accelerates the expression of LDL-receptors, followed by the uptake of LDL-C from blood to the liver, leading to a decrease in plasma LDL-C and total cholesterol. Sustained inhibition of cholesterol synthesis in the liver also decreases levels of very-low-density lipoproteins. The maximum LDL-C reduction of rosuvastatin tablets is usually achieved by 4 weeks and is maintained after that.
12.3 Pharmacokinetics
Absorption
In clinical pharmacology studies in man, peak plasma concentrations of rosuvastatin were reached 3 to 5 hours following oral dosing. Both C maxand AUC increased in approximate proportion to rosuvastatin tablets dose. The absolute bioavailability of rosuvastatin is approximately 20%. The AUC of rosuvastatin does not differ following evening or morning drug administration.Effect of food
Administration of rosuvastatin tablets with food did not affect the AUC of rosuvastatin.Distribution
Mean volume of distribution at steady-state of rosuvastatin is approximately 134 liters. Rosuvastatin is 88% bound to plasma proteins, mostly albumin. This binding is reversible and independent of plasma concentrations.Elimination
Metabolism
Rosuvastatin is not extensively metabolized; approximately 10% of a radiolabeled dose is recovered as metabolite. The major metabolite is N-desmethyl rosuvastatin, which is formed principally by cytochrome P450 2C9, and in vitrostudies have demonstrated that N-desmethyl rosuvastatin has approximately one-sixth to one-half the HMG‑CoA reductase inhibitory activity of the parent compound. Overall, greater than 90% of active plasma HMG‑CoA reductase inhibitory activity is accounted for by the parent compound.Excretion
Following oral administration, rosuvastatin and its metabolites are primarily excreted in the feces (90%). After an intravenous dose, approximately 28% of total body clearance was via the renal route, and 72% by the hepatic route. The elimination half-life of rosuvastatin is approximately 19 hours.Specific Populations
Geriatric Patients
There were no differences in plasma concentrations of rosuvastatin between the nonelderly and elderly populations (age ≥65 years).Pediatric Patients
In a population pharmacokinetic analysis of two pediatric trials involving patients with HeFH 10 to 17 years of age and 8 to 17 years of age, respectively, rosuvastatin exposure appeared comparable to or lower than rosuvastatin exposure in adult patients.Male and Female Patients
There were no differences in plasma concentrations of rosuvastatin between males and females.Racial or Ethnic Groups
A population pharmacokinetic analysis revealed no clinically relevant differences in pharmacokinetics among White, Hispanic or Latino ethnicity, and Black or Afro-Caribbean groups. However, pharmacokinetic studies, including one conducted in the US, have demonstrated an approximate 2‑fold elevation in median exposure (AUC and C max) in Asian subjects when compared with a White control group.Patients with Renal Impairment
Mild to moderate renal impairment (CL cr≥30 mL/min/1.73 m 2) had no influence on plasma concentrations of rosuvastatin. However, plasma concentrations of rosuvastatin increased to a clinically significant extent (about 3-fold) in patients with severe renal impairment (CL cr<30 mL/min/1.73 m 2) not receiving hemodialysis compared with healthy subjects (CL cr>80 mL/min/1.73 m 2).Steady-state plasma concentrations of rosuvastatin in patients on chronic hemodialysis were approximately 50% greater compared with healthy volunteer subjects with normal renal function.
Patients with Hepatic Impairment
In patients with chronic alcohol liver disease, plasma concentrations of rosuvastatin were modestly increased.In patients with Child‑Pugh A disease, C maxand AUC were increased by 60% and 5%, respectively, as compared with patients with normal liver function. In patients with Child‑Pugh B disease, C maxand AUC were increased 100% and 21%, respectively, compared with patients with normal liver function.
Drug Interaction studies
Rosuvastatin clearance is not dependent on metabolism by cytochrome P450 3A4 to a clinically significant extent.Rosuvastatin is a substrate for certain transporter proteins including the hepatic uptake transporter organic anion-transporting polyprotein 1B1 (OATP1B1) and efflux transporter breast cancer resistance protein (BCRP). Concomitant administration of rosuvastatin tablets with medications that are inhibitors of these transporter proteins (e.g., cyclosporine, certain HIV protease inhibitors) [see Dosage and Administration (2.6)and Drug Interactions (7.1)] and ticagrelor [see Drug Interactions (7.1)] may result in increased rosuvastatin plasma concentrations.
Table 8. Effect of Coadministered Drugs on Rosuvastatin Systemic Exposure
Coadministered drug and dosing regimen Rosuvastatin Mean Ratio
(ratio with/without coadministered drug)
No Effect=1.0Dose (mg) 1 Change in AUC Change in C max Sofosbuvir/velpatasvir/voxilaprevir (400 mg/100 mg/100 mg) + Voxilaprevir (100 mg) once daily for 15 days
10 mg, single dose
7.39 2
(6.68 to 8.18) 3
18.88 2
(16.23 to 21.96) 3
Cyclosporine – stable dose required (75 mg – 200 mg BID)
10 mg, QD for 10 days
7.1 2
11 2
Darolutamide 600 mg BID, 5 days
5 mg, single dose
5.2 2
~5 2
Regorafenib 160 mg QD, 14 days
5 mg, single dose
3.8 2
4.6 2
Atazanavir/ritonavir combination 300 mg/100 mg QD for 8 days
10 mg
3.1 2
7 2
Simeprevir 150 mg QD, 7 days
10 mg, single dose
2.8 2
(2.3 to 3.4) 3
3.2 2
(2.6 to 3.9) 3
Velpatasvir 100 mg once daily
10 mg, single dose
2.69 2
(2.46 to 2.94) 3
2.61 2
(2.32 to 2.92) 3
Ombitasvir 25 mg/paritaprevir 150 mg/ ritonavir 100 mg + dasabuvir 400 mg BID
5 mg, single dose
2.59 2
(2.09 to 3.21) 3
7.13 2
(5.11 to 9.96) 3
Teriflunomide
Not available
2.51 2
2.65 2
Enasidenib 100 mg QD, 28 days
10 mg, single dose
2.44
3.66
Elbasvir 50 mg/grazoprevir 200 mg once daily
10 mg, single dose
2.26 2
(1.89 to 2.69) 3
5.49 2
(4.29 to 7.04) 3
Glecaprevir 400 mg/pibrentasvir 120 mg once daily
5 mg, once daily
2.15 2
(1.88 to 2.46) 3
5.62 2
(4.80 to 6.59) 3
Lopinavir/ritonavir combination 400 mg/100 mg BID for 17 days
20 mg, QD for 7 days
2.1 2
(1.7 to 2.6) 3
5 2
(3.4 to 6.4) 3
Capmatinib 400 mg BID
10 mg, single dose
2.08 2
(1.56 to 2.76) 3
3.04 2
(2.36 to 3.92) 3
Fostamatinib 100 mg BID
20 mg, single dose
1.96 2
(1.77 to 2.15) 3
1.88 2
(1.69 to 2.09) 3
Febuxostat 120 mg QD for 4 days
10 mg, single dose
1.9 2
(1.5 to 2.5) 3
2.1 2
(1.8 to 2.6) 3
Gemfibrozil 600 mg BID for 7 days
80 mg
1.9 2
(1.6 to 2.2) 3
2.2 2
(1.8 to 2.7) 3
Tafamidis 61 mg BID on Days 1 & 2, followed by QD on Days 3 to 9
10 mg
1.97 2
(1.68 to 2.31) 3
1.86 2
(1.59 to 2.16) 3
Eltrombopag 75 mg QD, 5 days
10 mg
1.6
(1.4 to 1.7) 3
2
(1.8 to 2.3) 3
Darunavir 600 mg/ritonavir 100 mg BID, 7 days
10 mg, QD for 7 days
1.5
(1.0 to 2.1) 3
2.4
(1.6 to 3.6) 3
Tipranavir/ritonavir combination 500 mg/200 mg BID for 11 days
10 mg
1.4
(1.2 to 1.6) 3
2.2
(1.8 to 2.7) 3
Dronedarone 400 mg BID
10 mg
1.4
Itraconazole 200 mg QD, 5 days
10 mg or 80 mg
1.4
(1.2 to 1.6) 3
1.3
(1.1 to 1.4) 3
1.4
(1.2 to 1.5) 3
1.2
(0.9 to 1.4) 3
Ezetimibe 10 mg QD, 14 days
10 mg, QD for 14 days
1.2
(0.9 to 1.6) 31.2
(0.8-1.6) 3
Fosamprenavir/ritonavir 700 mg/100 mg BID for 7 days
10 mg
1.1
1.5
Fenofibrate 67 mg TID for 7 days
10 mg
↔
1.2
(1.1 to 1.3) 3
Rifampicin 450 mg QD, 7 days
20 mg
↔
Aluminum & magnesium hydroxide combination antacid
Administered simultaneously
Administered 2 hours apart40 mg
40 mg0.5 2
(0.4 to 0.5) 3
0.8(0.7 to 0.9) 3
0.5 2
(0.4 to 0.6) 3
0.8(0.7 to 1.0) 3
Ketoconazole 200 mg BID for 7 days
80 mg
1.0
(0.8 to 1.2) 3
1.0
(0.7 to 1.3) 3
Fluconazole 200 mg QD for 11 days
80 mg
1.1
(1.0 to 1.3) 3
1.1
(0.9 to 1.4) 3
Erythromycin 500 mg QID for 7 days
80 mg
0.8
(0.7 to 0.9) 3
0.7
(0.5 to 0.9) 3
QD= Once daily, BID= Twice daily, TID= Three times daily, QID= Four times daily
1Single dose unless otherwise noted.
2Clinically significant [see Dosage and Administration (2)and Warnings and Precautions (5)]
3Mean ratio with 90% CI (with/without coadministered drug, e.g., 1= no change, 0.7 = 30% decrease, 11=11-fold increase in exposure)Table 9. Effect of Rosuvastatin Coadministration on Systemic Exposure to Other Drugs
Rosuvastatin Dosage Regimen Coadministered Drug Mean Ratio
(ratio with/without coadministered drug)
No Effect = 1.0Name and Dose Change in AUC Change in C max 40 mg QD for 10 days
Warfarin 1
25 mg single dose
R-Warfarin
1.0
(1.0 to 1.1) 2
S-Warfarin
1.1
(1.0 to 1.1) 2
R-Warfarin
1.0
(0.9 to 1.0) 2
S-Warfarin
1.0
(0.9 to 1.1) 2
40 mg QD for 12 days
Digoxin
0.5 mg single dose
1.0
(0.9 to 1.2) 2
1.0
(0.9 to 1.2) 2
40 mg QD for 28 days
Oral Contraceptive
(ethinyl estradiol 0.035 mg & norgestrel 0.180, 0.215 and 0.250 mg) QD for 21 Days
EE 1.3
(1.2 to 1.3) 2
NG 1.3
(1.3 to 1.4) 2
EE 1.3
(1.2 to 1.3) 2
NG 1.2
(1.1 to 1.3) 2
EE = ethinyl estradiol, NG = norgestrel, QD= Once daily
1Clinically significant pharmacodynamic effects [see Drug Interactions (7.3)]
2Mean ratio with 90% CI (with/without coadministered drug, e.g., 1= no change, 0.7=30% decrease,11=11-fold increase in exposure)12.5 Pharmacogenomics
Disposition of rosuvastatin, involves OATP1B1 and other transporter proteins. Higher plasma concentrations of rosuvastatin have been reported in very small groups of patients (n=3 to 5) who have two reduced function alleles of the gene that encodes OATP1B1 ( SLCO1B1521T > C). The frequency of this genotype (i.e., SLCO1B1521 C/C) is generally lower than 5% in most racial/ethnic groups. The impact of this polymorphism on efficacy and/or safety of rosuvastatin tablets has not been clearly established.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 104‑week carcinogenicity study in rats at dose levels of 2, 20, 60, or 80 mg/kg/day by oral gavage, the incidence of uterine stromal polyps was significantly increased in females at 80 mg/kg/day at systemic exposure 20 times the human exposure at 40 mg/day based on AUC. Increased incidence of polyps was not seen at lower doses.
In a 107‑week carcinogenicity study in mice given 10, 60, or 200 mg/kg/day by oral gavage, an increased incidence of hepatocellular adenoma/carcinoma was observed at 200 mg/kg/day at systemic exposures 20 times the human exposure at 40 mg/day based on AUC. An increased incidence of hepatocellular tumors was not seen at lower doses.
Rosuvastatin was not mutagenic or clastogenic with or without metabolic activation in the Ames test with Salmonella typhimuriumand Escherichia coli,the mouse lymphoma assay, and the chromosomal aberration assay in Chinese hamster lung cells. Rosuvastatin was negative in the in vivomouse micronucleus test.
In rat fertility studies with oral gavage doses of 5, 15, 50 mg/kg/day, males were treated for 9 weeks prior to and throughout mating and females were treated 2 weeks prior to mating and throughout mating until gestation day 7. No adverse effect on fertility was observed at 50 mg/kg/day (systemic exposures up to 10 times the human exposure at 40 mg/day based on AUC). In testicles of dogs treated with rosuvastatin at 30 mg/kg/day for one month, spermatidic giant cells were seen. Spermatidic giant cells were observed in monkeys after 6‑month treatment at 30 mg/kg/day in addition to vacuolation of seminiferous tubular epithelium. Exposures in the dog were 20 times and in the monkey 10 times the human exposure at 40 mg/day based on body surface area. Similar findings have been seen with other drugs in this class.
-
14 CLINICAL STUDIES
Primary Prevention of CV Disease
In the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) study, the effect of rosuvastatin tablets on the occurrence of major CV disease events was assessed in 17,802 males (≥50 years) and females (≥60 years) who had no clinically evident CV disease, LDL-C levels <130 mg/dL and hsCRP levels ≥2 mg/L. The study population had an estimated baseline coronary heart disease risk of 11.6% over 10 years based on the Framingham risk criteria and included a high percentage of patients with additional risk factors such as hypertension (58%), low HDL-C levels (23%), cigarette smoking (16%), or a family history of premature CHD (12%). Patients had a median baseline LDL-C of 108 mg/dL and hsCRP of 4.3 mg/L. Patients were randomly assigned to placebo (n=8901) or rosuvastatin tablets 20 mg once daily (n=8901) and were followed for a mean duration of 2 years. The JUPITER study was stopped early by the Data Safety Monitoring Board due to meeting predefined stopping rules for efficacy in rosuvastatin tablets-treated subjects.The primary end point was a composite end point consisting of the time-to-first occurrence of any of the following major CV events: CV death, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina or an arterial revascularization procedure.
Rosuvastatin tablets significantly reduced the risk of major CV events (252 events in the placebo group vs. 142 events in the rosuvastatin group) with a statistically significant (p<0.001) relative risk reduction of 44% and absolute risk reduction of 1.2% (see Figure 1). The risk reduction for the primary end point was consistent across the following predefined subgroups: age, sex, race, smoking status, family history of premature CHD, body mass index, LDL‑C, HDL‑C, and hsCRP levels.
Figure 1. Time to First Occurrence of Major CV Events in JUPITER
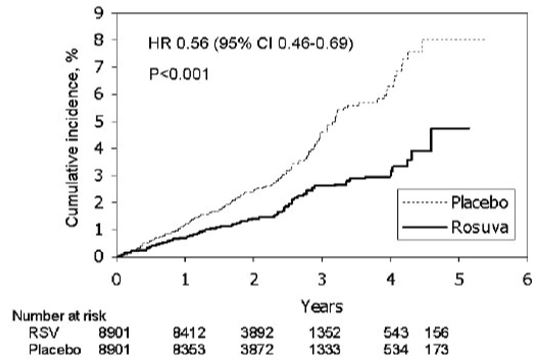
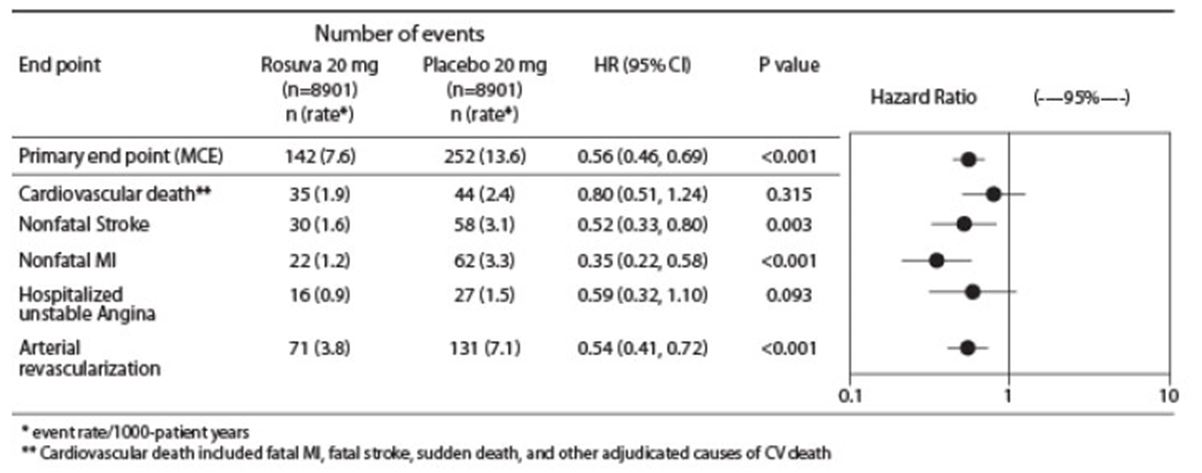
The individual components of the primary end point are presented in Figure 3. Rosuvastatin tablets significantly reduced the risk of nonfatal myocardial infarction, nonfatal stroke, and arterial revascularization procedures. There were no significant treatment differences between the rosuvastatin tablets and placebo groups for death due to CV causes or hospitalizations for unstable angina.
Rosuvastatin tablets significantly reduced the risk of myocardial infarction (6 fatal events and 62 nonfatal events in placebo-treated subjects vs. 9 fatal events and 22 nonfatal events in rosuvastatin tablets-treated subjects) and the risk of stroke (6 fatal events and 58 nonfatal events in placebo-treated subjects vs. 3 fatal events and 30 nonfatal events in rosuvastatin tablets-treated subjects).
In a post-hoc subgroup analysis of JUPITER subjects (rosuvastatin=725, placebo=680) with a hsCRP ≥2 mg/L and no other traditional risk factors (smoking, BP ≥140/90 or taking antihypertensives, low HDL‑C) other than age, after adjustment for high HDL‑C, there was no significant treatment benefit with rosuvastatin tablets treatment.
Figure 2. Major CV Events by Treatment Group in JUPITER
At one year, rosuvastatin tablets increased HDL-C and reduced LDL-C, hsCRP, total cholesterol and serum triglyceride levels (p<0.001 for all versus placebo).
Primary Hyperlipidemia in Adults
Rosuvastatin tablets reduces Total‑C, LDL-C, ApoB, non-HDL-C, and TG, and increases HDL-C, in adult patients with hyperlipidemia and mixed dyslipidemia.In a multicenter, double-blind, placebo-controlled study in patients with hyperlipidemia, rosuvastatin tablets given as a single daily dose (5 to 40 mg) for 6 weeks significantly reduced Total-C, LDL-C, non-HDL-C, and ApoB, across the dose range (Table 10).
Table 10: Lipid-modifying Effect of Rosuvastatin Tablets in Adult Patients with Hyperlipidemia (Adjusted Mean % Change from Baseline at Week 6)
Dose N Total-C LDL-C Non-HDL-C ApoB TG HDL-C Placebo
13
-5
-7
-7
-3
-3
3
Rosuvastatin tablets 5 mg
17
-33
-45
-44
-38
-35
13
Rosuvastatin tablets 10 mg
17
-36
-52
-48
-42
-10
14
Rosuvastatin tablets 20 mg
17
-40
-55
-51
-46
-23
8
Rosuvastatin tablets 40 mg
18
-46
-63
-60
-54
-28
10
Rosuvastatin tablets was compared with the statins (atorvastatin, simvastatin, and pravastatin) in a multicenter, open-label, dose‑ranging study of 2,240 patients with hyperlipidemia or mixed dyslipidemia. After randomization, patients were treated for 6 weeks with a single daily dose of either rosuvastatin tablets, atorvastatin, simvastatin, or pravastatin (Figure 3 and Table 11).
Figure 3. Percent LDL‑C Change by Dose of Rosuvastatin Tablets, Atorvastatin, Simvastatin, and Pravastatin at Week 6 in Adult Patients with Hyperlipidemia or Mixed Dyslipidemia
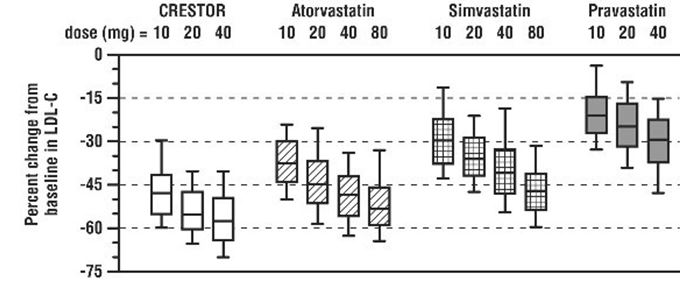
Box plots are a representation of the 25th, 50th, and 75th percentile values, with whiskers representing the 10th and 90th percentile values. Mean baseline LDL‑C: 189 mg/dL.
Table 11: Percent Change in LDL-C by Dose of Rosuvastatin Tablets, Atorvastatin, Simvastatin, and Pravastatin From Baseline to Week 6 (LS Mean 1) in Adult Patients with Hyperlipidemia or Mixed Dyslipidemia (Sample Sizes Ranging from 156–167 Patients Per Group)
Treatment Daily Dose
Treatment
10 mg
20 mg
40 mg
80 mg
Rosuvastatin
-46 2
-52 3
-55 4
---
Atorvastatin
-37
-43
-48
-51
Simvastatin
-28
-35
-39
-46
Pravastatin
-20
-24
-30
---
1Corresponding standard errors are approximately 1.00.
2Rosuvastatin tablets 10 mg reduced LDL-C significantly more than atorvastatin 10 mg; pravastatin 10 mg, 20 mg, and 40 mg; simvastatin 10 mg, 20 mg, and 40 mg. (p<0.002)
3Rosuvastatin tablets 20 mg reduced LDL-C significantly more than atorvastatin 20 mg and 40 mg; pravastatin 20 mg and 40 mg; simvastatin 20 mg, 40 mg, and 80 mg. (p<0.002)
4Rosuvastatin tablets 40 mg reduced LDL-C significantly more than atorvastatin 40 mg; pravastatin 40 mg; simvastatin 40 mg, and 80 mg. (p<0.002)Slowing of the Progression of Atherosclerosis
In the Measuring Effects on Intima Media Thickness: an Evaluation Of Rosuvastatin 40 mg (METEOR) study, the effect of therapy with rosuvastatin tablets on carotid atherosclerosis was assessed by B-mode ultrasonography in patients with elevated LDL‑C, at low risk (Framingham risk <10% over ten years) for symptomatic coronary artery disease and with subclinical atherosclerosis as evidenced by carotid intimal-medial thickness (cIMT). In this double-blind, placebo-controlled clinical study 984 adult patients were randomized (of whom 876 were analyzed) in a 5:2 ratio to rosuvastatin tablets 40 mg or placebo once daily. Ultrasonograms of the carotid walls were used to determine the annualized rate of change per patient from baseline to two years in mean maximum cIMT of 12 measured segments. The estimated difference in the rate of change in the maximum cIMT analyzed over all 12 carotid artery sites between patients treated with rosuvastatin tablets and placebo-treated patients was -0.0145 mm/year (95% CI –0.0196, –0.0093; p<0.0001).The annualized rate of change from baseline for the placebo group was +0.0131 mm/year (p<0.0001). The annualized rate of change from baseline for the group treated with rosuvastatin tablets was -0.0014 mm/year (p=0.32).
At an individual patient level in the group treated with rosuvastatin tablets, 52.1% of patients demonstrated an absence of disease progression (defined as a negative annualized rate of change), compared to 37.7% of patients in the placebo group.
HeFH in Adults
In a study of adult patients with HeFH (baseline mean LDL of 291 mg/dL), patients were randomized to rosuvastatin tablets 20 mg or atorvastatin 20 mg. The dose was increased at 6-week intervals. Significant LDL-C reductions from baseline were seen at each dose in both treatment groups (Table 12).Table 12: LDL-C Percent Change from Baseline
Rosuvastatin tablets (n=435)
LS Mean 1(95% CI)Atorvastatin (n=187)
LS Mean1(95% CI)Week 6
20 mg
-47% (-49%, -46%)
-38% (-40%, -36%)
Week 12
40 mg
-55% (-57%, -54%)
-47% (-49%, -45%)
Week 18
80 mg
NA
-52% (-54%, -50%)
1LS Means are least square means adjusted for baseline LDL-C
HeFH in Pediatric Patients
In a double-blind, randomized, multicenter, placebo-controlled, 12-week study, 176 (97 male and 79 female) pediatric patients with HeFH were randomized to rosuvastatin 5 mg, 10 mg or 20 mg or placebo daily. Patients ranged in age from 10 to 17 years (median age of 14 years) with approximately 30% of the patients 10 to 13 years and approximately 17%, 18%, 40%, and 25% at Tanner stages II, III, IV, and V, respectively. Females were at least 1 year postmenarche. Mean LDL-C at baseline was 233 mg/dL (range of 129 to 399). The 12‑week double-blind phase was followed by a 40 week open label dose-titration phase, where all patients (n=173) received 5 mg, 10 mg or 20 mg rosuvastatin daily.Rosuvastatin significantly reduced LDL-C (primary end point), total cholesterol and ApoB levels at each dose compared to placebo. Results are shown in Table 13 below.
Table 13: Lipid-Modifying Effects of Rosuvastatin Tablets in Pediatric Patients 10 to 17 years of Age with HeFH (Least-Squares Mean Percent Change from Baseline To Week 12)
Dose (mg) N LDL‑C HDL‑C Total‑C TG 1 ApoB Placebo
46
-1%
+7%
0%
-7%
-2%
5
42
-38%
+4% 2
-30%
-13% 2
-32%
10
44
-45%
+11% 2
-34%
-15% 2
-38%
20
44
-50%
+9% 2
-39%
16% 2
-41%
1Median percent change
2Difference from placebo not statistically significantRosuvastatin was also studied in a two-year open-label, uncontrolled, titration-to-goal trial that included 175 pediatric patients with HeFH who were 8 to 17 years old (79 males and 96 females). All patients had a documented genetic defect in the LDL receptor or in ApoB. Approximately 89% were White, 7% were Asian, 1% were Black or African American, and fewer than 1% were Hispanic or Latino ethnicity. Mean LDL-C at baseline was 236 mg/dL. Fifty-eight (33%) patients were prepubertal at baseline. The starting rosuvastatin dosage for all pediatric patients was 5 mg once daily. Pediatric patients aged 8 to less than 10 years (n=41 at baseline) could titrate to a maximum dosage of 10 mg once daily, and pediatric patients aged 10 to 17 years could titrate to a maximum dosage of 20 mg once daily.
The reductions in LDL‑C from baseline were generally consistent across age groups within the trial as well as with previous experience in both adult and pediatric controlled trials.
HoFH in Adult and Pediatric Patients
In an open-label, forced-titration study, HoFH patients (n=40, 8‑63 years) were evaluated for their response to rosuvastatin tablets 20 to 40 mg titrated at a 6‑week interval. In the overall population, the mean LDL‑C reduction from baseline was 22%. About one-third of the patients benefited from increasing their dose from 20 mg to 40 mg with further LDL‑C lowering of greater than 6%. In the 27 patients with at least a 15% reduction in LDL‑C, the mean LDL‑C reduction was 30% (median 28% reduction). Among 13 patients with an LDL‑C reduction of <15%, 3 had no change or an increase in LDL‑C. Reductions in LDL‑C of 15% or greater were observed in 3 of 5 patients with known receptor negative status.HoFH in Pediatric Patients
Rosuvastatin tablets was studied in a randomized, double-blind, placebo-controlled, multicenter, cross-over study in 14 pediatric patients with HoFH. The study included a 4‑week dietary lead‑in phase during which patients received rosuvastatin tablets 10 mg daily, a cross‑over phase that included two 6‑week treatment periods with either rosuvastatin tablets 20 mg or placebo in random order, followed by a 12‑week open‑label phase during which all patients received rosuvastatin tablets 20 mg. Patients ranged in age from 7 to 15 years of age (median 11 years), 50% were male, 71% were White, 21% were Asian, 7% were Black or African American, and no patients were of Hispanic or Latino ethnicity. Fifty percent were on apheresis therapy and 57% were taking ezetimibe. Patients who entered the study on apheresis therapy or ezetimibe continued the treatment throughout the entire study. Mean LDL‑C at baseline was 416 mg/dL (range 152 to 716 mg/dL). A total of 13 patients completed both treatment periods of the randomized cross-over phase; one patient withdrew consent due to inability to have blood drawn during the cross-over phase.Rosuvastatin tablets 20 mg significantly reduced LDL-C, total cholesterol, ApoB, and non-HDL-C compared to placebo (Table 14).
Table 14: Lipid-modifying Effects of Rosuvastatin Tablets in Pediatric Patients 7 to 15 years of Age with HoFH After 6 Weeks
Placebo
(N=13)
Rosuvastatin tablets 20 mg
(N=13)
Percent difference
(95% CI)LDL-C (mg/dL)
481
396
-22.3% (-33.5, -9.1) 1
Total-C (mg/dL)
539
448
-20.1% (-29.7, -9.1) 2
Non-HDL-C (mg/dL)
505
412
-22.9% (-33.7, -10.3) 2
ApoB (mg/dL)
268
235
-17.1% (-29.2, -2.9) 3
% Difference estimates are based on transformations of the estimated mean difference in log LDL measurements between rosuvastatin tablets and placebo using a mixed model adjusted for study period
1p=0.005, 2p=0.003, 3p=0.024Primary Dysbetalipoproteinemia in Adults
In a randomized, multicenter, double-blind crossover study, 32 patients (27 with є2/є2 and 4 with apo E mutation [Arg145Cys] with primary dysbetalipoproteinemia entered a 6‑week dietary lead-in period on the NCEP Therapeutic Lifestyle Change (TLC) diet. Following dietary lead-in, patients were randomized to a sequence of treatments for 6 weeks each: rosuvastatin 10 mg followed by rosuvastatin 20 mg or rosuvastatin 20 mg followed by rosuvastatin 10 mg. Rosuvastatin tablets reduced non HDL‑C (primary end point) and circulating remnant lipoprotein levels. Results are shown in the table below.Table 15: Lipid-modifying Effects of Rosuvastatin Tablets 10 mg and 20 mg in Adult Patients with Primary Dysbetalipoproteinemia (Type III hyperlipoproteinemia) After Six Weeks by Median Percent Change (95% CI) from Baseline (N=32)
Median at Baseline (mg/dL) Median percent change from baseline (95% CI)
Rosuvastatin tablets 10 mgMedian percent change from baseline (95% CI)
Rosuvastatin tablets 20 mgTotal‑C
342.5
-43.3
(-46.9, - 37.5)
-47.6
(-51.6, -42.8)
Triglycerides
503.5
-40.1
(-44.9, -33.6)
-43.0
(-52.5, -33.1)
Non-HDL‑C
294.5
-48.2
(-56.7, -45.6)
-56.4
(-61.4, -48.5)
VLDL‑C + IDL‑C
209.5
-46.8
(-53.7, -39.4)
-56.2
(-67.7, -43.7)
LDL‑C
112.5
-54.4
(-59.1, -47.3)
-57.3
(-59.4, -52.1)
HDL‑C
35.5
10.2
(1.9, 12.3)
11.2
(8.3, 20.5)
RLP‑C
82.0
-56.4
(-67.1, -49.0)
-64.9
(-74.0, -56.6)
Apo‑E
16.0
-42.9
(-46.3, -33.3)
-42.5
(-47.1, -35.6)
Hypertriglyceridemia in Adults
In a double-blind, placebo-controlled study in adult patients with baseline TG levels from 273 to 817 mg/dL, rosuvastatin tablets given as a single daily dose (5 to 40 mg) over 6 weeks significantly reduced serum TG levels (Table 16).Table 16: Lipid-Modifying Effect of Rosuvastatin Tablets in Adult Patients with Primary Hypertriglyceridemia After Six Weeks by Median (Min, Max) Percent Change from Baseline to Week 6
Dose Placebo
(n=26)Rosuvastatin tablets
5 mg
(n=25)Rosuvastatin tablets
10 mg
(n=23)Rosuvastatin tablets
20 mg
(n=27)Rosuvastatin tablets
40 mg
(n=25)Triglycerides
1 (-40, 72)
-21 (-58, 38)
-37 (-65, 5)
-37 (-72, 11)
-43 (-80, -7)
Non-HDL-C
2 (-13, 19)
-29 (-43, -8)
-49 (-59, -20)
-43 (-74, 12)
-51 (-62, -6)
Total-C
1 (-13, 17)
-24 (-40, -4)
-40 (-51, -14)
-34 (-61, -11)
-40 (-51, -4)
LDL-C
5 (-30, 52)
-28 (-71, 2)
-45 (-59, 7)
-31 (-66, 34)
-43 (-61, -3)
HDL-C
-3 (-25, 18)
3 (-38, 33)
8 (-8, 24)
22 (-5, 50)
17 (-14, 63)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Rosuvastatin Tablets USP are supplied as follows:
Strength
How Supplied
NDC
Tablet Description
5 mg
Bottle of 30 tablets
NDC: 60290-043-01
Pink, round, biconvex, beveled edge, film coated tablets debossed with “R5” on one side and plain on other side.
Bottle of 90 tablets
NDC: 60290-043-02
Bottle of 500 tablets
NDC: 60290-043-03
Blister pack of 100 unit-dose tablets
NDC: 60290-043-04
Bottle of 1000 tablets
NDC: 60290-043-05
10 mg
Bottle of 30 tablets
NDC: 60290-044-01
Pink, round, biconvex, beveled edge, film coated tablets debossed with “R10” on one side and plain on other side.
Bottle of 90 tablets
NDC: 60290-044-02
Bottle of 500 tablets
NDC: 60290-044-03
Blister pack of 100 unit-dose tablets
NDC: 60290-044-04
Bottle of 1000 tablets
NDC: 60290-044-05
20 mg
Bottle of 30 tablets
NDC: 60290-045-01
Pink, round, biconvex, film coated tablets debossed with “R20” on one side and plain on other side.
Bottle of 90 tablets
NDC: 60290-045-02
Bottle of 500 tablets
NDC: 60290-045-03
Blister pack of 100 unit-dose tablets
NDC: 60290-045-04
Bottle of 1000 tablets
NDC: 60290-045-05
40 mg
Bottle of 30 tablets
NDC: 60290-046-01
Pink, oval, biconvex, film-coated tablets debossed with “R40” on one side and plain on other side.
Bottle of 90 tablets
NDC: 60290-046-02
Bottle of 500 tablets
NDC: 60290-046-03
Blister pack of 100 unit-dose tablets
NDC: 60290-046-04
Bottle of 1000 tablets
NDC: 60290-046-05
Storage
Store at controlled room temperature, 20°C to 25ºC (68°F to 77ºF); excursions permitted between 15ºC and 30ºC (59°F and 86ºF) [see USP Controlled Room Temperature]. Protect from moisture.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling ( Patient Information).
Myopathy and Rhabdomyolysis
Advise patients that rosuvastatin tablets may cause myopathy and rhabdomyolysis. Inform patients that the risk is also increased when taking certain types of medication and they should discuss all medication, both prescription and over the counter, with their healthcare provider. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever [see Warnings and Precautions (5.1), and Drug Interactions (7.1)].
Hepatic Dysfunction
Inform patients that rosuvastatin tablets may cause liver enzyme elevations and possibly liver failure. Advise patients to promptly report fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice [see Warnings and Precautions (5.3)].
Inform patients that increases in HbA1c and fasting serum glucose levels may occur with rosuvastatin tablets. Encourage patients to optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices [see Warnings and Precautions (5.5)].
Pregnancy
Advise pregnant patients and patients who can become pregnant of the potential risk to a fetus. Advise patients to inform their healthcare provider of a known or suspected pregnancy to discuss if rosuvastatin tablets should be discontinued [see Use in Specific Populations (8.1)].
Lactation
Advise patients that breastfeeding during treatment with rosuvastatin tablets is not recommended [see Use in Specific Populations (8.2)].
Concomitant Use of Antacids
When taking rosuvastatin tablets with an aluminum and magnesium hydroxide combination antacid, administer rosuvastatin tablets at least 2 hours at least 2 hours before the antacid [see Drug Interactions (7.2)].
Missed Doses
If a dose is missed, advise patients not to take an extra dose. Just resume the usual schedule [see Dosage and Administration Information (2.1)].
Manufactured by:
Umedica Laboratories Pvt. Ltd.
Vapi, Gujarat 396195, INDIA (IND).Distributed by:
Umedica Laboratories USA Inc.
Parsippany, NJ, 07054Product of India
Revised: 12/25, V-00
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
ROSUVASTATIN (roe soo” va stat’ in)
tablets, USP for oral useRead this Patient Information carefully before you start taking rosuvastatin tablets and each time you get a refill. If you have any questions about rosuvastatin tablets, ask your healthcare provider. Only your healthcare provider can determine if rosuvastatin tablets are right for you.
What are rosuvastatin tablets?
Rosuvastatin tablets are a prescription medicine that contains a cholesterol-lowering medicine called rosuvastatin.
- Rosuvastatin tablets is used:
- to reduce the risk of major adverse cardiovascular (CV) events, such as death from cardiovascular disease, heart attack, stroke, or the need for procedures to improve blood flow to the heart called arterial revascularization in adults who do not have known heart disease but do have certain additional risk factors.
- along with diet to:
- lower the level of low-density lipoprotein (LDL-C) cholesterol or “bad” cholesterol in adults with primary hyperlipidemia.
- slow the buildup of fatty deposits (plaque) in the walls of blood vessels.
- treat adults and children 8 years of age and older with high blood cholesterol due to heterozygous familial hypercholesterolemia (HeFH) (an inherited condition that causes high levels of LDL-C).
- along with other cholesterol lowering treatments or alone if such treatments are unavailable in adults and children 7 years of age and older with homozygous familial hypercholesterolemia (HoFH) (an inherited condition that causes high levels of LDL-C).
- along with diet for the treatment of adults with:
- primary dysbetalipoproteinemia (an inherited condition that causes high levels of cholesterol and fat).
- hypertriglyceridemia.
It is not known if rosuvastatin tablets is safe and effective in children younger than 8 years of age with HeFH or children younger than 7 years of age with HoFH or in children with other types of hyperlipidemias (other than HeFH or HoFH).
Do not take rosuvastatin tablets if you:
- have liver problems.
- are allergic to rosuvastatin or any of the ingredients in rosuvastatin tablets. See the end of this leaflet for a complete list of ingredients in rosuvastatin tablets.
Before you take rosuvastatin tablets, tell your healthcare provider about all of your medical conditions, including if you:
- have unexplained muscle aches or weakness.
- have or have had kidney problems.
- have or have had liver problems.
- drink more than 2 glasses of alcohol daily.
- have thyroid problems.
- are of Asian descent.
- are pregnant or think you may be pregnant, or are planning to become pregnant. If you become pregnant while taking rosuvastatin tablets, call your healthcare provider right away to discuss your rosuvastatin tablets treatment.
- are breastfeeding. Rosuvastatin can pass into your breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take rosuvastatin tablets. Do not breastfeed while taking rosuvastatin tablets.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Tell your healthcare provider who prescribes rosuvastatin tablets if another healthcare provider increases the dose of another medicine you are taking. Rosuvastatin tablets may affect the way other medicines work, and other medicines may affect how rosuvastatin tablets works.
Especially tell your healthcare provider if you take:
- coumarin anticoagulants (medicines that prevent blood clots, such as warfarin)
- antacids (medicines you take for heartburn that contain aluminum and magnesium hydroxide
Taking rosuvastatin tablets with certain medicines may increase the risk of muscle problems.
Especially tell your healthcare provider if you take:
- cyclosporine (a medicine for your immune system)
- teriflunomide (a medicine used to treat relapsing remitting multiple sclerosis)
- enasidenib (a medicine used to treat acute myeloid leukemia)
- capmatinib (a medicine for the treatment of non-small cell lung cancer)
- fostamatinib (a medicine used to treat low platelet counts)
- febuxostat (a medicine used to treat and prevent high blood levels of uric acid)
- gemfibrozil (a fibric acid medicine for lowering cholesterol)
- tafamidis [used to treat cardiomyopathy (enlarged and thickened heart muscle)]
- anti-viral medicines including certain HIV or hepatitis C virus drugs such as:
- lopinavir, ritonavir, fosamprenavir, tipranavir, atazanavir, simeprevir
- combination of
- sofosbuvir/velpatasvir/voxilaprevir
- dasabuvir/ombitasvir/paritaprevir/ritonavir
- elbasvir/grazoprevir
- sofosbuvir/velpatasvir
- glecaprevir/pibrentasvir and
- all other combinations with ledipasvir including ledipasvir/sofosbuvir
- darolutamide (a medicine for the treatment of prostate cancer)
- regorafenib (a medicine used to treat cancer of the colon and rectum)
- fibric acid derivatives (such as fenofibrate)
- ticagrelor (helps reduce the chance of a blood clot formation that can block a blood vessel)
- niacin or nicotinic acid
- colchicine (a medicine used to treat gout)
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get new medicine.
How should I take rosuvastatin tablets?
- Take rosuvastatin tablets exactly as your healthcare provider tells you to take it.
- Take rosuvastatin tablets, by mouth, 1 time each day, with or without food Swallow the tablet whole.
- Rosuvastatin tablets can be taken at any time of day, with or without food.
- Do notchange your dose or stop rosuvastatin tablets without talking to your healthcare provider, even if you are feeling well.
- Your healthcare provider may do blood tests to check your cholesterol levels before and during your treatment with rosuvastatin tablets. Your healthcare provider may change your dose of rosuvastatin tablets if needed.
- While taking rosuvastatin tablets, continue to follow your cholesterol-lowering diet and to exercise as your healthcare provider told you to.
- If you take a medicine called an antacid that contains a combination of aluminum and magnesium hydroxide, take rosuvastatin tablets at least 2 hours before you take the antacid.
- If you miss a dose of rosuvastatin tablets, take your next dose at your normal scheduled time. Do not takean extra dose of rosuvastatin tablets.
- In case of an overdose, get medical help or contact a live Poison Center expert right away at 1-800-222-1222. Advice is also available online at poisonhelp.org.
What are the possible side effects of rosuvastatin tablets?
Rosuvastatin tablets may cause serious side effects, including:
-
Muscle pain, tenderness and weakness (myopathy). Muscle problems, including muscle breakdown, can be serious in some people and rarely cause kidney damage that can lead to death. Tell your healthcare provider right away if:
- you have unexplained muscle pain, tenderness, or weakness, especially if you have a fever or feel more tired than usual, while you take rosuvastatin tablets.
- you have muscle problems that do not go away even after your healthcare provider has told you to stop taking rosuvastatin tablets. Your healthcare provider may do further tests to diagnose the cause of your muscle problems.
Your chances of getting muscle problems are higher if you:
- are taking certain other medicines while you take rosuvastatin tablets (see “Especially tell your healthcare provider if you take”)
- are 65 years of age or older
- are of Asian descent
- have thyroid problems (hypothyroidism) that are not controlled
- have kidney problems
- are taking higher doses of rosuvastatin tablets
-
Liver problems.Your healthcare provider may do blood tests to check your liver before you start taking rosuvastatin tablets and if you have symptoms of liver problems while you take rosuvastatin tablets. Call your healthcare provider right away if you have any of the following symptoms of liver problems:
- feel unusually tired or weak
- loss of appetite
- upper belly pain
- dark urine
- yellowing of your skin or the whites of your eyes
- Protein and blood in the urine.Rosuvastatin tablets may cause you to have protein and blood in your urine. If you develop protein or blood in your urine, your healthcare provider may decrease your dose of rosuvastatin tablets.
- Increase in blood sugar (glucose) levels. Rosuvastatin tablets may cause an increase in your blood sugar levels.
The most common side effects may include headache, nausea, muscle aches and pains, weakness, and constipation.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store rosuvastatin tablets?
- Store rosuvastatin tablets at room temperature, between 68°F to 77°F (20°C to 25°C) and in a dry place.
Keep rosuvastatin tablets and all medicines out of the reach of children.
General Information about the safe and effective use of rosuvastatin tablets
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use rosuvastatin tablets for a condition for which it was not prescribed. Do not give rosuvastatin tablets to other people, even if they have the same medical condition you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about rosuvastatin tablets that is written for health professionals.
What are the Ingredients in rosuvastatin tablets?
Active Ingredient:rosuvastatin as rosuvastatin calcium USP
Inactive Ingredients:crospovidone USP, hypromellose USP, iron oxide red NF, lactose monohydrate USP, magnesium stearate USP, microcrystalline cellulose USP, sodium bicarbonate powder NF, titanium dioxide USP, triacetin USP.
Trademarks are the property of their respective owners.
Manufactured by:
Umedica Laboratories Pvt. Ltd.
Vapi, Gujarat 396195, India.
Distributed by:
Umedica Laboratories USA Inc.
Parsippany, NJ, 07054.
Product of India
Rev: 12/25, V-00
This Patient Information has been approved by the U.S. Food and Drug Administartion.
- Rosuvastatin tablets is used:
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL
Rosuvastatin Tablets, USP 5 mg - NDC: 60290-043-01 - 30s Bottle Label
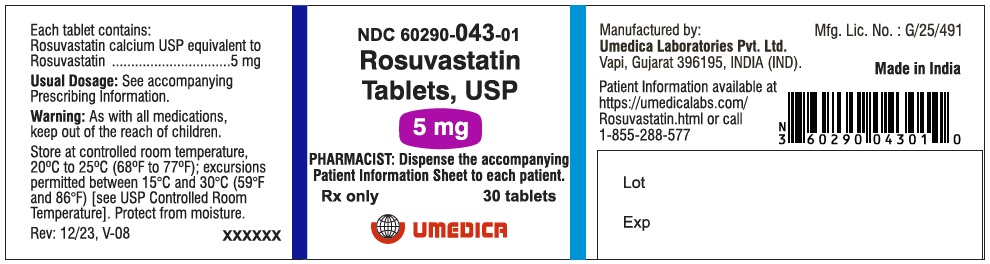
Rosuvastatin Tablets, USP 10 mg - NDC: 60290-044-01 - 30s Bottle Label

Rosuvastatin Tablets, USP 20 mg - NDC: 60290-045-01 - 30s Bottle Label
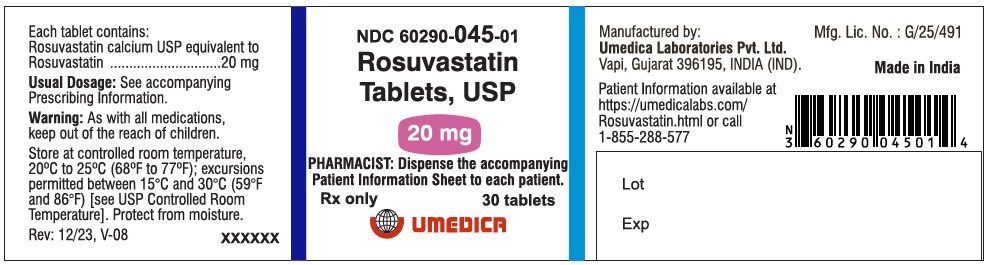
Rosuvastatin Tablets, USP 40 mg - NDC: 60290-046-01 - 30s Bottle Label
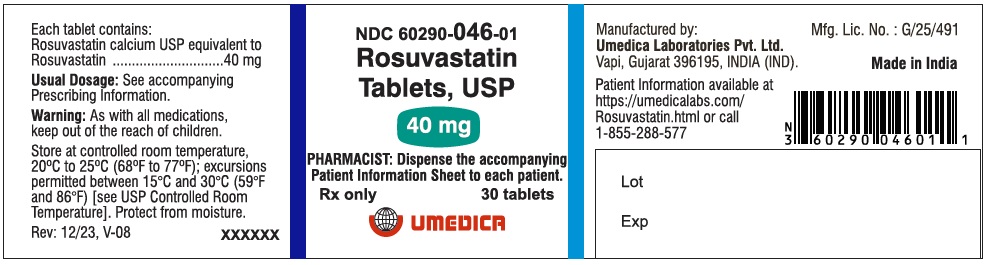
-
INGREDIENTS AND APPEARANCE
ROSUVASTATIN CALCIUM
rosuvastatin calcium tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 60290-044 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROSUVASTATIN CALCIUM (UNII: 83MVU38M7Q) (ROSUVASTATIN - UNII:413KH5ZJ73) ROSUVASTATIN 10 mg Inactive Ingredients Ingredient Name Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color pink Score no score Shape ROUND Size 5mm Flavor Imprint Code R10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60290-044-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2026 2 NDC: 60290-044-02 90 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2025 3 NDC: 60290-044-03 500 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2025 4 NDC: 60290-044-04 100 in 1 BLISTER PACK; Type 0: Not a Combination Product 02/15/2026 5 NDC: 60290-044-05 1000 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207626 11/30/2025 ROSUVASTATIN CALCIUM
rosuvastatin calcium tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 60290-043 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROSUVASTATIN CALCIUM (UNII: 83MVU38M7Q) (ROSUVASTATIN - UNII:413KH5ZJ73) ROSUVASTATIN 5 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) FERRIC OXIDE RED (UNII: 1K09F3G675) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SODIUM BICARBONATE (UNII: 8MDF5V39QO) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color pink Score no score Shape ROUND Size 4mm Flavor Imprint Code R5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60290-043-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2026 2 NDC: 60290-043-02 90 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2025 3 NDC: 60290-043-03 500 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2025 4 NDC: 60290-043-04 100 in 1 BLISTER PACK; Type 0: Not a Combination Product 02/15/2026 5 NDC: 60290-043-05 1000 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207626 11/30/2025 ROSUVASTATIN CALCIUM
rosuvastatin calcium tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 60290-045 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROSUVASTATIN CALCIUM (UNII: 83MVU38M7Q) (ROSUVASTATIN - UNII:413KH5ZJ73) ROSUVASTATIN 20 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SODIUM BICARBONATE (UNII: 8MDF5V39QO) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color pink Score no score Shape ROUND Size 7mm Flavor Imprint Code R20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60290-045-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2026 2 NDC: 60290-045-02 90 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2025 3 NDC: 60290-045-03 500 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2025 4 NDC: 60290-045-04 100 in 1 BLISTER PACK; Type 0: Not a Combination Product 02/15/2026 5 NDC: 60290-045-05 1000 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207626 11/30/2025 ROSUVASTATIN CALCIUM
rosuvastatin calcium tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 60290-046 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROSUVASTATIN CALCIUM (UNII: 83MVU38M7Q) (ROSUVASTATIN - UNII:413KH5ZJ73) ROSUVASTATIN 40 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SODIUM BICARBONATE (UNII: 8MDF5V39QO) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color pink Score no score Shape OVAL Size 11mm Flavor Imprint Code R40 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60290-046-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2025 2 NDC: 60290-046-02 90 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2025 3 NDC: 60290-046-03 500 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2025 4 NDC: 60290-046-04 100 in 1 BLISTER PACK; Type 0: Not a Combination Product 02/15/2026 5 NDC: 60290-046-05 1000 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207626 11/30/2025 Labeler - Umedica Laboratories USA Inc. (119579082) Registrant - UMEDICA LABORATORIES PRIVATE LIMITED (920635096) Establishment Name Address ID/FEI Business Operations UMEDICA LABORATORIES PRIVATE LIMITED 920635096 analysis(60290-043, 60290-044, 60290-045, 60290-046) , manufacture(60290-043, 60290-044, 60290-045, 60290-046) , pack(60290-043, 60290-044, 60290-045, 60290-046)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.