EMADINE- emedastine difumarate solution/ drops
EMADINE by
Drug Labeling and Warnings
EMADINE by is a Prescription medication manufactured, distributed, or labeled by Alcon Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
EMADINE® (emedastine difumarate ophthalmic solution) 0.05% is a sterile ophthalmic solution containing emedastine, a relatively selective, H1-receptor antagonist for topical administration to the eyes. Emedastine difumarate is a white, crystalline, water-soluble fine powder with a molecular weight of 534.57. The chemical structure is presented below:
Structural Formula:
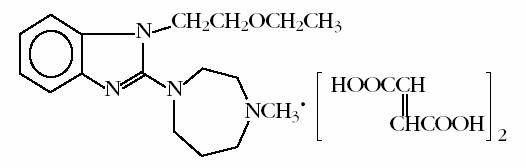
Chemical Name:
1H-benzimidazole,1-(2-ethoxyethyl)-2-(hexahydro-4-methyl-1H-1,4-diazepin-1-yl),(E)-2-butenedioate (1:2)
Each mL of EMADINE® (emedastine difumarate ophthalmic solution) 0.05% contains: Active: 0.884 mg emedastine difumarate equivalent to 0.5 mg emedastine. Preservative: benzalkonium chloride 0.01%. Inactives: tromethamine; sodium chloride; hypromellose; hydrochloric acid/sodium hydroxide (adjust pH); and purified water. It has a pH of approximately 7.4 and an osmolality of approximately 300 mOsm/kg.
-
CLINICAL PHARMACOLOGY
Emedastine is a relatively selective, histamine H1 antagonist. In vitro examinations of emedastine's affinity for histamine receptors (H1: Ki = 1.3 nM, H2: Ki = 49,067 nM, and H3: Ki = 12,430 nM) demonstrate relative selectivity for the H1-histamine receptor. In vivo studies have shown concentration-dependent inhibition of histamine-stimulated vascular permeability in the conjunctiva following topical ocular administration.
Emedastine appears to be devoid of effects on adrenergic, dopaminergic and serotonin receptors. Following topical administration in man, emedastine was shown to have low systemic exposure. In a study involving 10 normal volunteers dosed bilaterally twice daily for 15 days with emedastine ophthalmic solution 0.05%, plasma concentrations of the parent compound were generally below the quantitation limit of the assay (less than 0.3 ng/mL).
Samples in which emedastine was quantifiable, ranged from 0.30 to 0.49 ng/mL. The elimination half-life of oral emedastine in plasma is 3-4 hours. Approximately 44% of the oral dose is recovered in the urine over 24 hours with only 3.6% of the dose excreted as parent drug. Two primary metabolites, 5- and 6-hydroxyemedastine, are excreted in the urine as both free and conjugated forms. The 5’-oxoanalogs of 5- and 6-hydroxyemedastine and the N-oxide are also formed as minor metabolites.
In an environmental study, patients with allergic conjunctivitis were treated with EMADINE® (emedastine difumarate ophthalmic solution) 0.05% for six weeks. The results demonstrated that EMADINE® (emedastine difumarate ophthalmic solution) 0.05% provides relief of the signs and symptoms of allergic conjunctivitis. In conjunctival antigen challenge studies, in which subjects were challenged with antigen both initially and up to four hours after dosing, EMADINE® (emedastine difumarate ophthalmic solution) 0.05% was demonstrated to be significantly more effective than placebo in preventing ocular itching associated with allergic conjunctivitis.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Information for Patients:
To prevent contaminating the dropper tip and solution, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle. Keep the bottle tightly closed when not in use. Do not use if the solution has become discolored.
Patients should be advised not to wear a contact lens if their eye is red. EMADINE® (emedastine difumarate ophthalmic solution) 0.05% should not be used to treat contact lens related irritation. The preservative in EMADINE® (emedastine difumarate ophthalmic solution) 0.05%, benzalkonium chloride, may be absorbed by soft contact lenses. Patients who wear soft contact lenses and whose eyes are not red, should be instructed to wait at least ten minutes after instilling EMADINE® (emedastine difumarate ophthalmic solution) 0.05% before they insert their contact lenses.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Emedastine difumarate demonstrated no carcinogenicity effects in lifetime studies in mice and rats at dietary doses more than 80,000 times and more than 26,000 times the maximum recommended ocular human use level of 0.002 mg/kg/day for a 50 kg adult, respectively. Higher dose levels were not tested. Emedastine difumarate was determined to be nonmutagenic in an in vitro bacterial reverse mutation (Ames) test, an in vitro modification of the Ames test, an in vitro mammalian chromosome aberration test, an in vitro mammalian forward mutation test, an in vitro mammalian DNA repair synthesis test, an in vivo mammalian sister chromatid exchange test and an in vivo mouse micronucleus test. There was no evidence of impaired fertility or reproductive capacity in rats at 15,000 times the maximum recommended ocular human use level.
Pregnancy:
Teratology and peri- and post-natal studies have been conducted with emedastine difumarate in rats and rabbits. At 15,000 times the maximum recommended ocular human use level, emedastine difumarate was shown not to be teratogenic in rats and rabbits and no effects on peri/post-natal development were observed in rats. However, at 70,000 times the maximum recommended ocular human use level, emedastine difumarate was shown to increase the incidence of external, visceral and skeletal anomalies in rats. There are, however, no adequate and well controlled studies in pregnant women. Because animal studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
-
Nursing Mothers:
Emedastine has been identified in breast milk in rats following oral administration. It is not known whether topical ocular administration could result in sufficient systemic absorption to produce detectable quantities in breast milk. Nevertheless, caution should be exercised when EMADINE® (emedastine difumarate ophthalmic solution) 0.05% is administered to a nursing mother.
-
ADVERSE REACTIONS
In controlled clinical studies of EMADINE® (emedastine difumarate ophthalmic solution) 0.05% lasting for 42 days, the most frequent adverse reaction was headache 11%. The following adverse experiences were reported in less than 5% of patients: Abnormal dreams, asthenia, bad taste, blurred vision, burning or stinging, corneal infiltrates, corneal staining, dermatitis, discomfort, dry eye, foreign body sensation, hyperemia, keratitis, pruritus, rhinitis, sinusitis and tearing. Some of these events were similar to the underlying disease being studied.
- OVERDOSAGE
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
EMADINE® (emedastine difumarate ophthalmic solution) 0.05% is supplied as follows:
5 mL in opaque, plastic DROP-TAINER® dispenser.
5 mL: NDC: 0065-0325-05
STORAGE: Store at 4°C to 30°C (39°F-86°F).
Rx Only
©2002, 2003, 2009, 2018 Novartis
Distributed by:
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Alcon®
a Novartis company
Revised: April 2018
T2018-34
- PRINCIPLE DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EMADINE
emedastine difumarate solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0065-0325 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EMEDASTINE DIFUMARATE (UNII: 42MB94QOSM) (EMEDASTINE - UNII:9J1H7Y9OJV) EMEDASTINE 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) HYPROMELLOSES (UNII: 3NXW29V3WO) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0065-0325-05 1 in 1 CARTON 02/15/1998 1 5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020706 02/15/1998 Labeler - Alcon Laboratories, Inc. (008018525)
Trademark Results [EMADINE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EMADINE 75032887 2231037 Live/Registered |
NOVARTIS AG 1995-12-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
