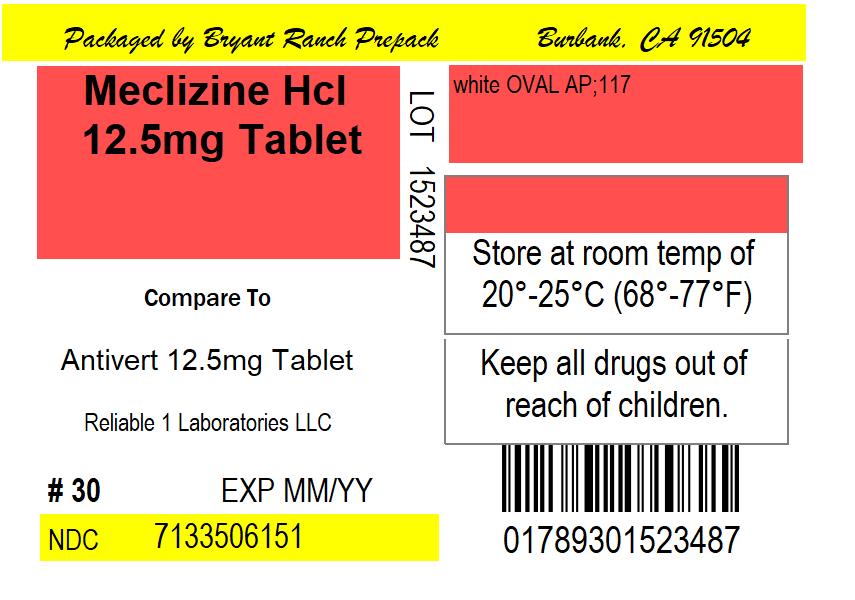
Meclizine HCL 12.5 mg by Bryant Ranch Prepack Meclizine HCL 12.5
Meclizine HCL 12.5 mg by
Drug Labeling and Warnings
Meclizine HCL 12.5 mg by is a Otc medication manufactured, distributed, or labeled by Bryant Ranch Prepack. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MECLIZINE HCL 12.5 MG- meclizine hcl 12.5 mg tablet
Bryant Ranch Prepack
----------
Meclizine HCL 12.5
Uses
- prevents and treats nausea, vomiting, or dizziness due to motion sickness
- for other uses, consult your doctor
Warnings
Ask a docotor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
When using this product
- you may get drowsy
- avoid alcoholic drinks
- alcohol, sedatives and tranquillizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
When using this prodcut
- you may get drowsy
- avoid alcoholic drinks
- alcohol, sedatives and tranqulizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- adults & children 12 years and over: 2-4 caplets once daily
- children under 12 years: ask a doctor
Other information
- each caplet contains: calcium 51 mg
- store at room temperature 15º-30ºC (59º-86ºF)
- This is a bulk package. Dispense contents with a child-resistant closure in a tight, light-resistant container as defined in the USP.
Inactive ingredients
croscarmellose sodium, dicalcium phosphate, magnesium stearate, microcrystalline cellulose, silicon dioxide, stearic acid
HOW SUPPLIED
NDC: 71335-0615-1: 30 Tablets in a BOTTLE, PLASTIC
NDC: 71335-0615-2: 60 Tablets in a BOTTLE, PLASTIC
NDC: 71335-0615-3: 90 Tablets in a BOTTLE, PLASTIC
NDC: 71335-0615-4: 28 Tablets in a BOTTLE, PLASTIC
NDC: 71335-0615-5: 20 Tablets in a BOTTLE, PLASTIC
NDC: 71335-0615-6: 120 Tablets in a BOTTLE, PLASTIC
NDC: 71335-0615-7: 100 Tablets in a BOTTLE, PLASTIC
| MECLIZINE HCL 12.5 MG
meclizine hcl 12.5 mg tablet |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Registrant - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(71335-0615) , RELABEL(71335-0615) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.