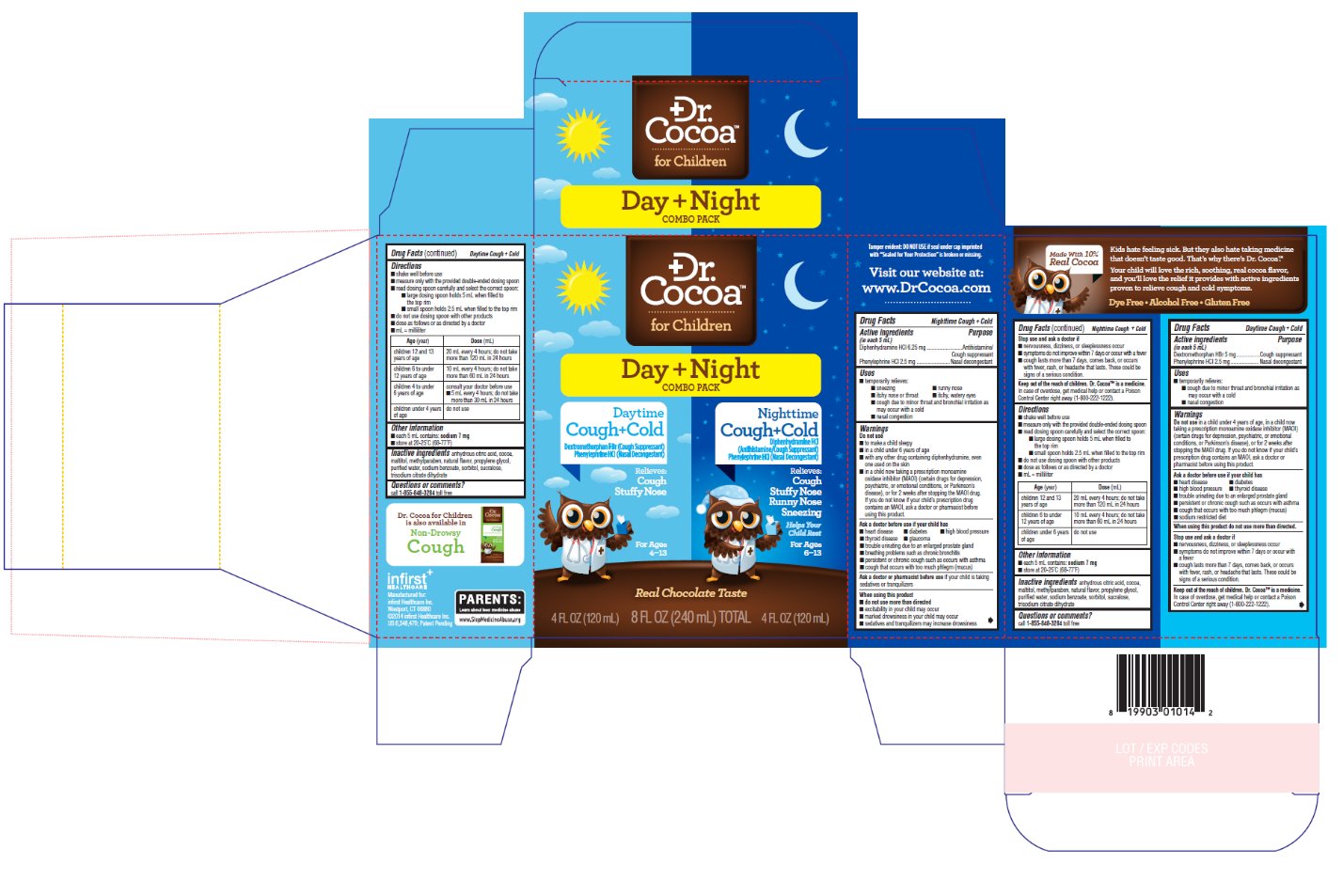
DR COCOA DAY NIGHT COMBO- dextromethorphan hydrobromide,diphenhydramine hydrochloride kit
Dr Cocoa by
Drug Labeling and Warnings
Dr Cocoa by is a Otc medication manufactured, distributed, or labeled by Infirst Healthcare. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Nighttime Cough + ColdDrug Facts
- Active ingredients
- Purpose
- Uses
-
Warnings
Do not use
- to make a child sleepy
- in a child under 6 years of age
- with any other drug containing diphenhydramine, even one used on the skin
- in a child now taking a prescription monoamine oxidase inhibitor (MAOI)(certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child’s prescription drug contains an MAOI, ask a doctor or pharmacist before using this product
Ask a doctor before use if your child has
- heart disease
- diabetes
- high blood pressure
- thyroid disease
- glaucoma
- trouble urinating due to an enlarged prostate gland
- breathing problems such as chronic bronchitis
- persistent or chronic cough such as occurs with asthma
- cough that occurs with too much phlegm (mucus)
Ask a doctor or pharmacist before use if your child is taking sedatives or tranquilizers
When using this product
- do not use more than directed
- excitability in your child may occur
- marked drowsiness in your child may occur
- sedatives and tranquilizers may increase drowsiness
-
Directions
- shake well before use
- measure only with the provided double-ended dosing spoon
-
read dosing spoon carefully and select the correct spoon:
- large dosing spoon holds 5 mL when filled to the top rim
- small spoon holds 2.5 mL when filled to the top rim
- do not use dosing spoon with other products
- dose as follows or as directed by a doctor
- mL=milliliter
Age (year)
Dose (mL)
children 12 and 13 years of age
20 mL every 4 hours; do not take more than 120 mL in 24 hours
children 6 to under 12 years of age
10 mL every 4 hours; do not take more than 60 mL in 24 hours
children under 6 years of age
do not use
- Other information
- Inactive ingredients
- Questions or comments?
- Daytime Cough + ColdDrug Facts
- Active ingredients
- Purpose
- Uses
-
Warnings
Do not use in a child under 4 years of age, in a child now taking a prescription monoamine oxidase inhibitor (MAOI)(certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child’s prescription drug contains an MAOI, ask a doctor or pharmacist before using this product
Ask a doctor before use if your child has
- heart disease
- diabetes
- high blood pressure
- thyroid disease
- trouble urinating due to an enlarged prostate gland
- persistent or chronic cough such as occurs with asthma
- cough that occurs with too much phlegm (mucus)
- sodium restricted diet
When using this product do not use more than directed.
-
Directions
- shake well before use
- measure only with the provided double-ended dosing spoon
-
read dosing spoon carefully and select the correct spoon:
- large dosing spoon holds 5 mL when filled to the top rim
- small spoon holds 2.5 mL when filled to the top rim
- do not use dosing spoon with other products
- dose as follows or as directed by a doctor
- mL=milliliter
Age (year)
Dose (mL)
children 12 and 13 years of age
20 mL every 4 hours; do not take more than 120 mL in 24 hours
children 6 to under 12 years of age
10 mL every 4 hours; do not take more than 60 mL in 24 hours
children 4 to under 6 years of age
consult your doctor before use
- 5 mL every 4 hours; do not take more than 30 mL in 24 hours
children under 4 years of age
do not use
- Other information
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL - 240 mL Carton
Dr.
Cocoa™
....................
for ChildrenDay + Night
Combo PackDaytime
Cough+ColdDextromethorphan HBr (Cough Suppressant)
Phenylephrine HCl (Nasal Decongestant)Relieves:
Cough
Stuffy NoseFor Ages 4-13
Nighttime
Cough+ColdDiphenhydramine HCl (Antihistamine/Cough Suppressant)
Phenylephrine HCl (Nasal Decongestant)Relieves:
Cough
Stuffy Nose
Runny Nose
Sneezing
Helps Your Child RestFor Ages 6-13
Real Chocolate Taste
4 FL OZ (120 mL) 8 FL OZ (240 mL) TOTAL 4 FL OZ (120 mL)
-
INGREDIENTS AND APPEARANCE
DR COCOA DAY NIGHT COMBO
dextromethorphan hydrobromide,diphenhydramine hydrochloride kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62372-732 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62372-732-04 1 in 1 CARTON; Type 0: Not a Combination Product 06/20/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 120 mL Part 2 1 BOTTLE 120 mL Part 1 of 2 DR. COCOA DAYTIME COUGH AND COLD
dextromethorphan hydrobromide, phenylephrine hydrochloride liquidProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 5 mg in 5 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 2.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) COCOA (UNII: D9108TZ9KG) MALTITOL (UNII: D65DG142WK) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 120 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 06/20/2014 Part 2 of 2 DR. COCOA NIGHTTIME COUGH AND COLD
diphenhydramine hydrochloride, phenylephrine hydrochloride liquidProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 6.25 mg in 5 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 2.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) COCOA (UNII: D9108TZ9KG) MALTITOL (UNII: D65DG142WK) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 120 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 06/20/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 06/20/2014 Labeler - Infirst Healthcare (079159739)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.