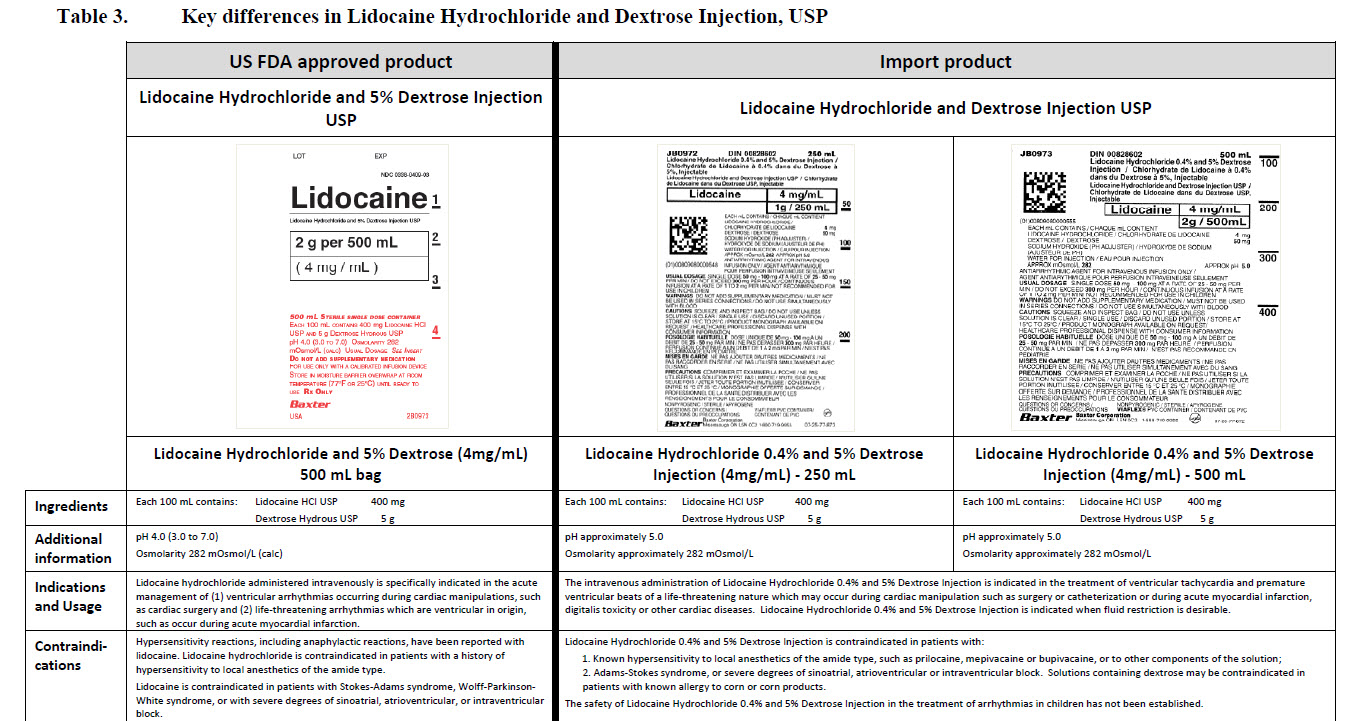
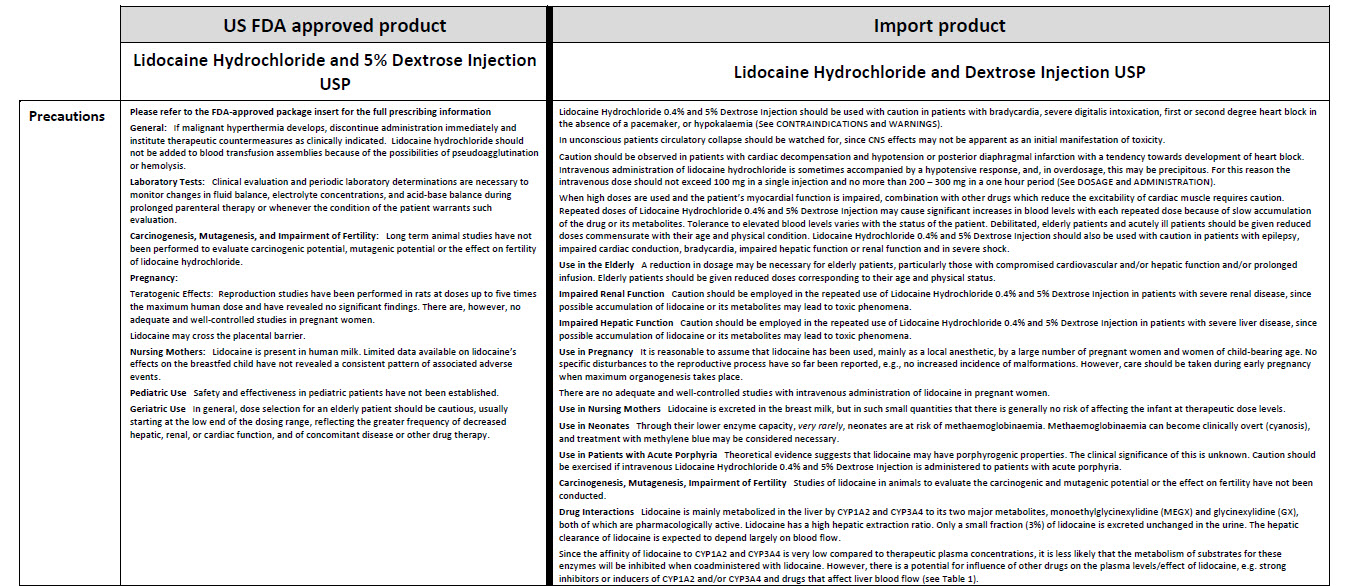
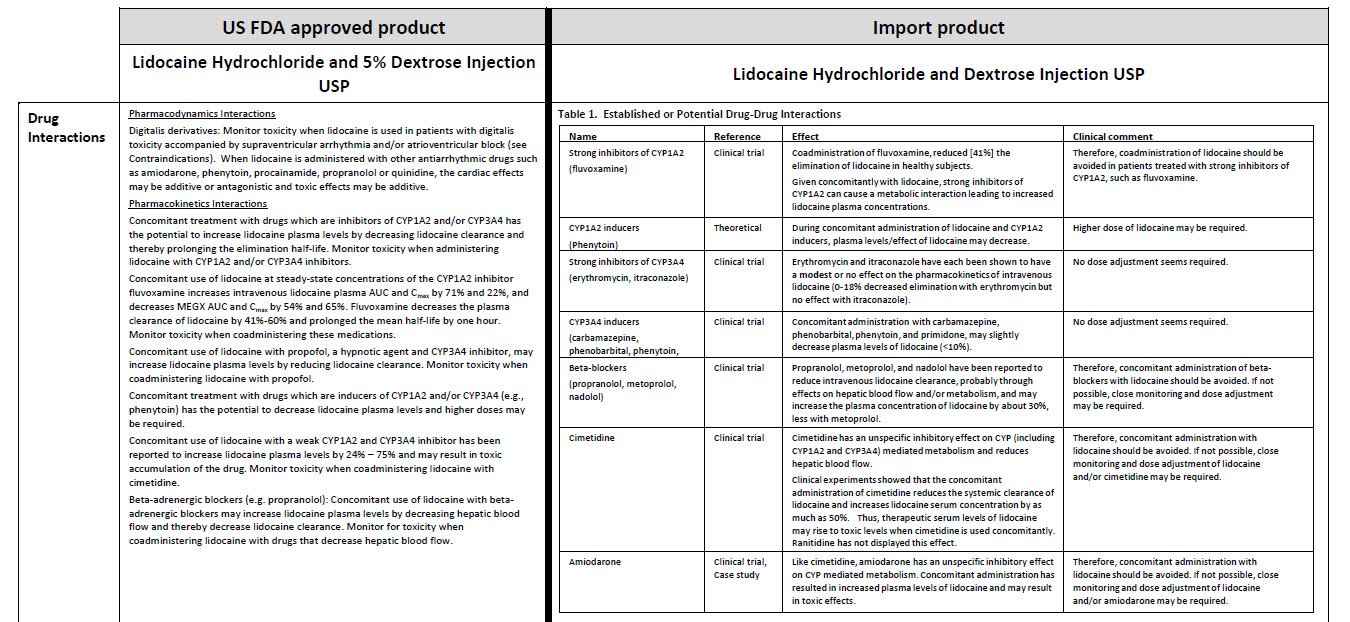
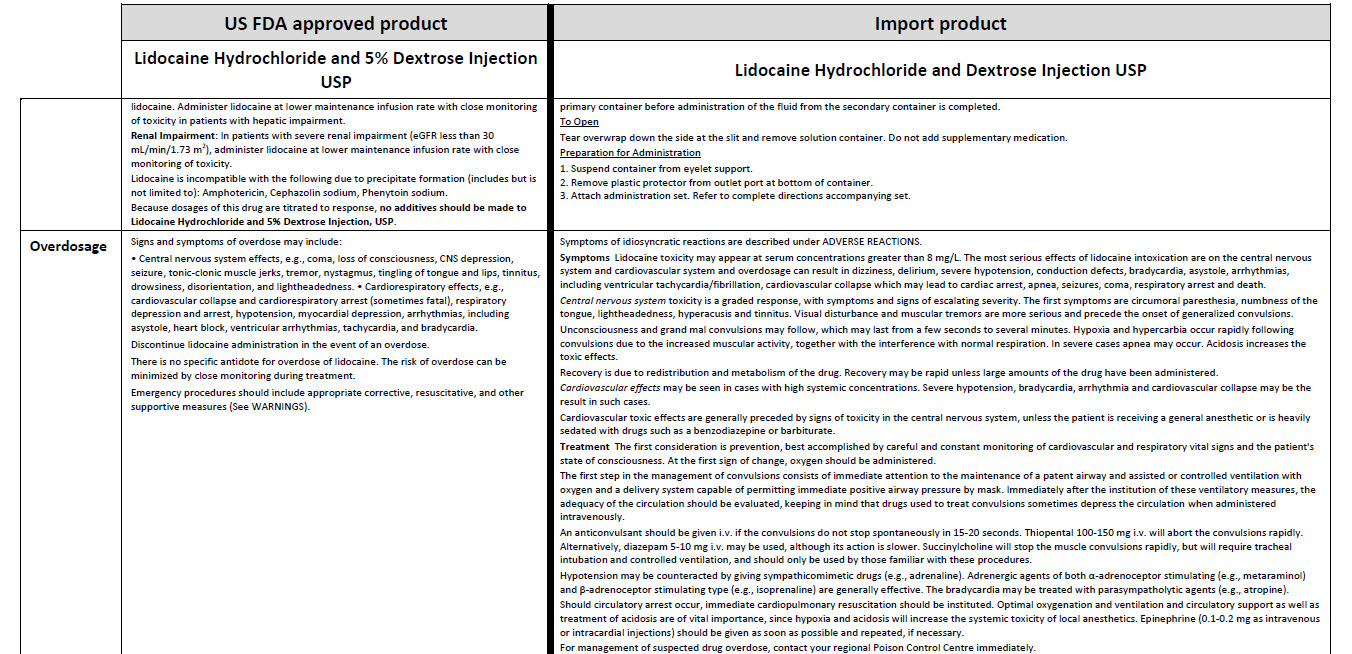
5% Dextrose Injection, USP In MINI-BAG Plus Container VIAFLEX Plastic Container
Dextrose by
Drug Labeling and Warnings
Dextrose by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation, Baxter Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DEXTROSE- dextrose monohydrate injection
Baxter Healthcare Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
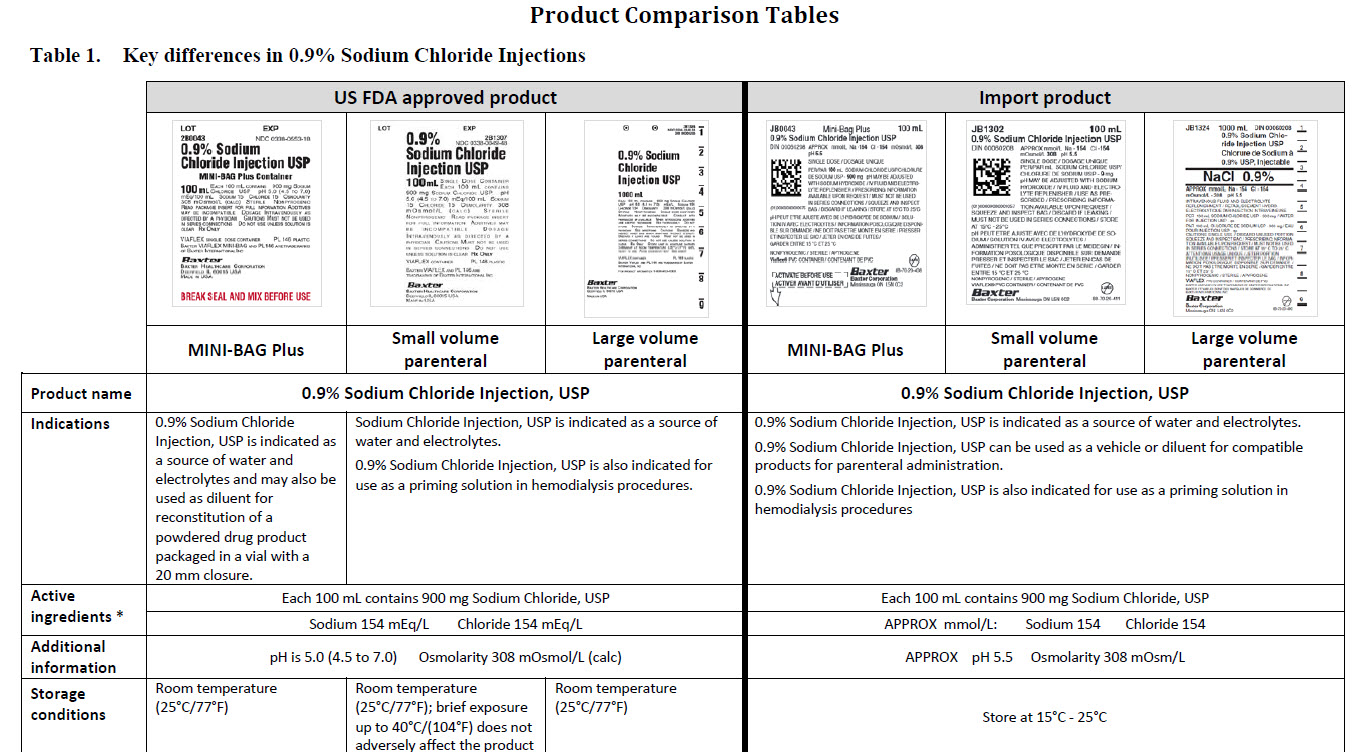
5% Dextrose Injection, USP
In MINI-BAG Plus Container
VIAFLEX Plastic Container
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
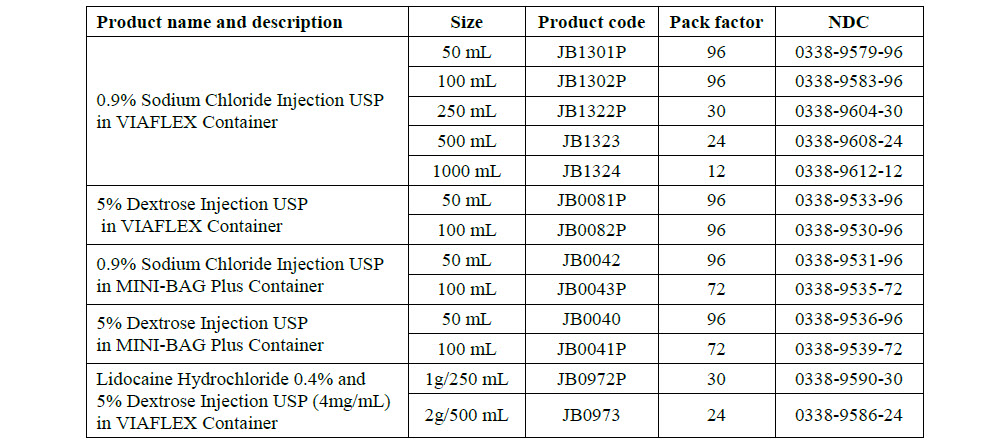
JB0040
50 mL
DIN 00060348
Mini-Bag Plus
5% Dextrose Injection USP
Bar Code
(01)00809080000944
APPROX mOsmol/L 252 pH 4.0 SINGLE DOSE / DOSAGE UNIQUE
PER/PAR 50 mL DEXTROSE HYDROUS USP / DEXTROSE HYDRATE USP –
2.5 g pH MAY BE ADJUSTED WITH SODIUM HYDROXIDE / IV FLUID NUTRI-
ENT REPLENISHER / DIRECTION SHEET AVAILABLE UPON REQUEST /
MUST NOT BE USED IN SERIES CONNECTIONS/ DO NOT ADMINISTER
SIMULTANEOUSLY WITH BLOOD / SQUEEZE AND INSPECT BAG / DISCARD
IF LEAKING / STORE AT 15ºC TO 25º C
pH PEUT ETRE AJUSTE AVEC DE L’HYDROXYDE DE SODIUM / SOLUTION IV POUR NUTRITION
PARENTERALE / FEUILLE DE MODE D’EMPLOI DISPONIBLE SUR DEMANDE / NE DOIT PAS ETRE
MONTE EN SERIE / NE PAS ADMINISTRER SIMULTANEMENT AVEC LE SANG / PRESSER ET
INSPECTER LE SAC / JETER EN CAS DE FUITES / GARDER ENTRE 15ºC ET 25 ºC
NONPYROGENIC / STERILE / APYROGENE
Viaflex® PVC CONTAINER / CONTENANT DE PVC
ACTIVATE BEFORE USE
ACTIVER AVANT D’UTILISER
Baxter Logo
Baxter Corporation
Mississauga ON L5N 0C2
No Latex Symbol
88-70-20-290
JB0041
100 Ml
DIN 00060348
Mini-Bag Plus
5% Dextrose Injection USP
Bar Code
(01)00809080000951
APPROX mOsmol/L 252 pH 4.0
SINGLE DOSE / DOSAGE UNIQUE
PER/PAR 100 mL DEXTROSE HYDROUS USP / DEX-
TROSE HYDRATE USP – 5 g pH MAY BE ADJUSTED
WITH SODIUM HYDROXIDE / IV FLUID NUTRIENT
REPLENISHER / DIRECTION SHEET AVAILABLE UPON
REQUEST / MUST NOT BE USED IN SERIES CONNECTIONS/ DO NOT
ADMINISTER SIMULTANEOUSLY WITH BLOOD / SQUEEZE AND INSPECT
BAG / DISCARD IF LEAKING / STORE AT 15ºC TO 25º C
pH PEUT ETRE AJUSTE AVEC DE L’HYDROXYDE DE SODIUM /
SOLUTION IV POUR NUTRITION PARENTERALE / FEUILLE DE MODE
D’EMPLOI DISPONIBLE SUR DEMANDE / NE DOIT PAS ETRE MONTE
EN SERIE / NE PAS ADMINISTRER SIMULTANEMENT AVEC LE SANG /
PRESSER ET INSPECTER LE SAC / JETER EN CAS DE FUITES /
GARDER ENTRE 15ºC ET 25 ºC
NONPYROGENIC / STERILE / APYROGENE
Viaflex® PVC CONTAINER / CONTENANT DE PVC
ACTIVATE BEFORE USE
ACTIVER AVANT D’UTILISER
Baxter Logo
Baxter Corporation
Mississauga ON L5N 0C2
No Latex Symbol
88-70-20-292
| DEXTROSE
dextrose monohydrate injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| DEXTROSE
dextrose monohydrate injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Corporation | 205087968 | ANALYSIS(0338-9536, 0338-9539) , MANUFACTURE(0338-9536, 0338-9539) , LABEL(0338-9536, 0338-9539) , PACK(0338-9536, 0338-9539) , STERILIZE(0338-9536, 0338-9539) | |