MAPAP- acetaminophen tablet
Mapap by
Drug Labeling and Warnings
Mapap by is a Otc medication manufactured, distributed, or labeled by Major Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if
- adult takes more than 4,000 mg in 24 hours
- child takes more than 5 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
-
DOSAGE & ADMINISTRATION
Directions
do not take more than directed (see overdose warning)
AGE DOSE Adults and children 12 years and over - take 2 tablets every 4 to 6 hours while symptoms last
- do not take more than 10 tablets in 24 hours, unless directed by a doctor
- do not use for more than 10 days unless directed by a doctor
Children 6 years to under 12 years - take 1 tablet every 4 to 6 hours while symptoms last
- do not take more than 5 tablets in 24 hours
- do not use for more than 5 days unless directed by a doctor
Children under 6 years ask a doctor - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- OTHER SAFETY INFORMATION
- SPL UNCLASSIFIED SECTION
-
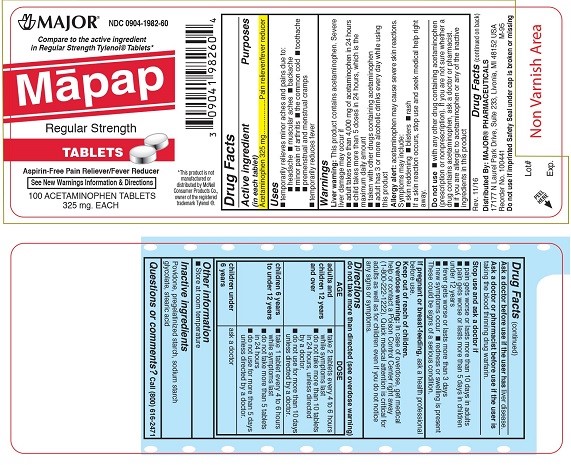
PRINCIPAL DISPLAY PANEL
MAJOR®
NDC: 0904-1982-60
Mapap
Regular Strength
TABLETS
Aspirin–Free Pain Reliever/Fever Reducer
See New Warnings Information & Directions
100 ACETAMINOPHEN TABLETS
325 mg EACH
NDC: 0904-1982-61
Unit Dose

-
INGREDIENTS AND APPEARANCE
MAPAP
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-1982 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength POVIDONE K12 (UNII: 333AG72FWJ) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) STEARIC ACID (UNII: 4ELV7Z65AP) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) Product Characteristics Color white Score no score Shape ROUND (flat faced beveled edge) Size 10mm Flavor Imprint Code GPI;A325 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-1982-59 1 in 1 BOX 04/21/2011 03/31/2021 1 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 0904-1982-61 100 in 1 CARTON 04/21/2011 10/31/2019 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 0904-1982-51 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/21/2011 06/30/2021 4 NDC: 0904-1982-60 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/21/2011 07/31/2021 5 NDC: 0904-1982-80 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/21/2011 09/30/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 04/21/2011 09/30/2021 Labeler - Major Pharmaceuticals (191427277)
Trademark Results [Mapap]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MAPAP 76126557 2490633 Live/Registered |
Harvard Drug Group, LLC, The 2000-09-12 |
 MAPAP 74215554 1732421 Dead/Cancelled |
MAJOR PHARMACEUTICAL CORPORATION 1991-10-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.