GUAIFENESIN DM- guaifenesin and dextromethorphan solution
Guaifenesin DM by
Drug Labeling and Warnings
Guaifenesin DM by is a Otc medication manufactured, distributed, or labeled by Pharmaceutical Associates, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Stop use and ask a doctor if cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- shake well before using
- do not take more than 6 doses in any 24-hour period
age dose adults and children 12 years and over 10 mL (2 teaspoonsful) every 4 hours children 6 to under 12 years of age 5 mL (1 teaspoonful) every 4 hours children 2 to under 6 years of age 2.5 mL (1/2 teaspoonful) every 4 hours children under 2 years consult a doctor
Other information
- each teaspoonful contains: sodium 4 mg
- store at 20° - 25°C (68° - 77°F)
- alcohol/sugar free
- red, cherry flavored solution supplied in the following oral dosage forms: NDC 0121-0809-04 (4 fl oz bottle), NDC 0121-0809-08 (8 fl oz bottle), NDC 0121-4809-05 (unit dose cups of 5 mL, packaged in trays of 10), and NDC 0121-4809-10 (unit dose cups of 10 mL, packaged in trays of 10).
- Inactive ingredients
- Questions or comments?
-
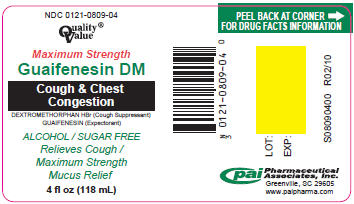
PRINCIPAL DISPLAY PANEL - 118 mL Label
NDC: 0121-0809-04
Quality®
ValueMaximum Strength
Guaifenesin DM
Cough & Chest
CongestionDEXTROMETHORPHAN HBr (Cough Suppressant)
GUAIFENESIN (Expectorant)ALCOHOL / SUGAR FREE
Relieves Cough /
Maximum Strength
Mucus Relief4 fl oz (118 mL)
PRINCIPAL DISPLAY PANEL - 5 mL Lid
Delivers 5 mL
NDC: 0121-4809-05MAXIMUM STRENGTH
GUAIFENESIN DMGuaifenesin (Expectorant)
Dextromethorphan HBr (Cough Suppressant)200 mg/10 mg per 5 mL
Alcohol Free / Sugar Free
FOR INSTITUTIONAL USE ONLY
PHARMACEUTICAL ASSOCIATES, INC.
GREENVILLE, SC 29605
SEE INSERT
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN DM
guaifenesin and dextromethorphan solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0121-0809 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 5 mL DEXTROMETHORPHAN (UNII: 7355X3ROTS) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN 10 mg in 5 mL Product Characteristics Color RED Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0121-0809-08 237 mL in 1 BOTTLE 2 NDC: 0121-0809-04 118 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 05/17/2010 GUAIFENESIN DM
guaifenesin and dextromethorphan solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0121-4809 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 5 mL DEXTROMETHORPHAN (UNII: 7355X3ROTS) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN 10 mg in 5 mL Product Characteristics Color RED Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0121-4809-05 4 in 1 CASE 1 10 in 1 TRAY 1 5 mL in 1 CUP, UNIT-DOSE 2 NDC: 0121-4809-10 4 in 1 CASE 2 10 in 1 TRAY 2 10 mL in 1 CUP, UNIT-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 05/17/2010 Labeler - Pharmaceutical Associates, Inc. (044940096) Establishment Name Address ID/FEI Business Operations Pharmaceutical Associates, Inc. 044940096 MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.