Salicylic Acid by Acella Pharmaceuticals, LLC SALICYLIC ACID gel
Salicylic Acid by
Drug Labeling and Warnings
Salicylic Acid by is a Prescription medication manufactured, distributed, or labeled by Acella Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
DESCRIPTION
Salicylic Acid 6% Gel is applied topically and used in the removal of excessive keratin in hyperkeratotic skin disorders. Each gram of Salicylic Acid 6% Gel contains salicylic acid 6% as the active ingredient, and the following inactive ingredients: propylene glycol, SD-40 alcohol (21%), hydroxypropylcellulose and purified water.
CHEMICAL STRUCTURE
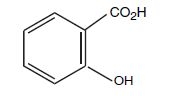
Salicylic acid is the 2-hydroxy derivative of benzoic acid having the following chemical structure:
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
Salicylic acid has been shown to produce desquamation of the horny layer of skin while not affecting qualitative or quantitative changes in structure of the viable epidermis. 1,2 The mechanism of action has been attributed to dissolution of intercellular cement substance. 3 In a study of the percutaneous absorption of salicylic acid from Salicylic Acid 6% Gel in four patients with extensive active psoriasis, Taylor and Halprin 4 showed that peak serum salicylate levels never exceeded 5 mg/100 mL even though more than 60% of the applied salicylic acid was absorbed. Systemic toxic reactions are usually associated with much higher serum levels (30 to 40 mg/100mL). Peak serum levels occurred within 5 hours of the topical application under occlusion. The sites were occluded for 10 hours over the entire body surface below the neck. Since salicylates are distributed in the extracellular space, patients with a contracted extracellular space due to dehydration or diuretics have higher salicylate levels than those with a normal extracellular space. 5 (See PRECAUTIONS).
The major metabolites identified in the urine after topical administration are salicyluric acid (52%), salicylate glucuronides (42%), and free salicylic acid (6%). 4 The urinary metabolites after percutaneous absorption differ from those after oral salicylate administration; those derived from percutaneous absorption contain more glucuronides and less salicyluric and salicylic acid. Almost 95% of a single dose of salicylate is excreted within 24 hours of its entrance into the extracellular space. 5
Fifty to eighty percent of salicylate is protein bound to albumin. Salicylates compete with the binding of several drugs and can modify the action of these drugs. By similar competetive mechanisms other drugs can influence the serum levels of salicylate. (See PRECAUTIONS).
- INDICATIONS & USAGE
- CONTRAINDICATIONS
-
WARNINGS
WARNINGS
Contact with eyes, lips, broken or inflamed skin, and all mucous membranes should be avoided.
Prolonged use over large areas, especially in children and those patients with significant renal or hepatic impairment could result in salicylism. Concomitant use of other drugs which may contribute to elevated serum salicylate levels should be avoided where the potential for toxicity is present. In children under 12 years of age and those patients with renal or hepatic impairment, the area to be treated should be limited and the patient monitored closely for signs of salicylate toxicity: nausea, vomiting, dizziness, loss of hearing, tinnitus, lethargy, hyperpnoea, diarrhea, psychic disturbances. In the event of salicylic acid toxicity, the use of Salicylic Acid 6% Gel should be discontinued. Fluids should be administered to promote urinary excretion. Treatment with sodium bicarbonate (oral or intravenous) should be instituted as appropriate.
Considering the potential of developing Reye’s syndrome, salicylate products should not be administered to children or teenagers with varicella or influenza, unless directed by a physician.
-
PRECAUTIONS
PRECAUTIONS
For external use only. Avoid contact with eyes and other mucous membranes. Mild burning or stinging may occur. Peeling of the skin may increase as the salicylic acid works to loosen excess keratin. If excessive burning, stinging or peeling occurs, discontinue use and consult your physician. Flammable. Keep away from heat and open flame. Keep this and all medications out of the reach of children.
-
DRUG INTERACTIONS
Drug Interactions. (The following interactions are from a published review 5 and include reports concerning both oral and topical salicylate administration. The relationship of these interactions to the use of Salicylic Acid 6% Gel is not known.)
I. Due to the competition of salicylate with other drugs for binding to serum albumin the following drug interactions may occur:
Drug Description of Interaction Tolbutamide; Sulfonylureas Hypoglycemia potentiated Methotrexate Decrease tubular reabsorption; clinical toxicity from methotrexate can result Oral Anticoagulants Increased bleeding
II. Drugs changing salicylate levels by altering renal tubular reabsorption:Drug Description of Interaction Corticosteroids Decreases plasma salicylate level; tapering doses of steroids may promote salicylism Ammonium Sulfate Increases plasma salicylate level
III. Drugs with complicated interactions with salicylates:Drug Description of Interaction Heparin Salicylate decreases platelet adhesivesness and interferes with hemostasis in heparin-treated patients Pyrazinamide Inhibits pyrazinamide-induced hyperuricemia Uricosuric Agents Effect of probenecid, sulfinpyrazone and phenylbutazone inhibited
The following alterations of laboratory tests have been reported during salicylate therapy 6:Laboratory Tests Effect of Salicylates Thyroid Function Decreased PBI; increased T 3 uptake Urinary Sugar False negative with glucose oxidase; false positive with Clinitest with high-dose salicylate therapy (2 - 5 g qd) 5 Hydroxyindole Acetic Acid False negative with fluorometric test Acetone, Ketone Bodies False positive FeCl 3 in Gerhardt reaction; red color persists with boiling 17-OH Corticosteroids False reduced values with >4.8 g qd salicylate Vanilmandelic Acid False reduced values Uric Acid May increase or decrease depending on dose Prothrombin Decreased levels; slightly increased prothrombin time -
PREGNANCY
Pregnancy (Category C) -Salicylic acid has been shown to be teratogenic in rats and monkeys. It is difficult to extrapolate from oral doses of acetylsalicylic acid used in these studies to topical administration as the oral dose to monkeys may represent 4 times the maximum daily human dose of salicylic acid (as supplied in one tube, 40 g of Salicylic Acid 6% Gel) when applied topically over a large body surface. There are no adequate and well-controlled studies in pregnant women. Salicylic Acid 6% Gel should be used during pregnancy only if the potential benefit justifies the risk to the fetus.
Nursing Mothers - It is not known whether topically applied salicylic acid is excreted in human milk. Due to the fact that many drugs are excreted in human milk, caution should be exercised by physicians when administering Salicylic Acid 6% Gel to nursing mothers and nursing mothers should certainly not apply Salicylic Acid 6% Gel to the chest area or any other part of the body with which the nursing child’s mouth is likely to come in contact.
Because of the potential for serious advers reactions in nursing infants from the mother’s use of Salicylic Acid 6% Gel, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of Fertility - No data are available concerning potential carcinogenic or reproductive effects of Salicylic Acid 6% Gel. It has been shown to lack mutagenic potential in the Ames Salmonella test.
KEEP THIS AND ALL OTHER MEDICATIONS OUT OF THE REACH OF CHILDREN.
- ADVERSE REACTIONS
- OVERDOSAGE
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
The preferable method of use is to apply Salicylic Acid 6% Gel thoroughly to the affected area and occlude the area at night. Preferably, the skin should be hydrated for at least five minutes prior to application. The medication is washed off in the morning and if excessive drying and/or irritation is observed a bland cream or lotion may be applied. Once clearing is apparent, the occasional use of Salicylic Acid 6% Gel will usually maintain the remission. In those areas where occlusion is difficult or impossible, application may be made more frequently; hydration by wet packs or baths prior to application apparently enhances the effect. Unless hands are being treated, hands should be rinsed thoroughly after application.
- HOW SUPPLIED
- STORAGE AND HANDLING
-
REFERENCES
REFERENCES:
- Davies M, Marks R: Br J Dermatol 95: 187-192, 1976.
- Mars R, Davies M, Cattel A: J Invest Dermatol 64: 283, 1975.
- Huber C, Christophers E: Arch Derm Res 257: 293-297, 1977.
- Taylor JR, Halprin KM: Arch Dermatol 111: 740-743, 1975.
- Goldsmith LA: Int J Dermatol 18: 32-36.
- Wilson JG, Ritter EJ, Scott WJ, Fradlein R: Tox Appl Pharmacol 41: 67-78, 1977.
-
SPL UNCLASSIFIED SECTION
All prescription substitutions using this product shall be made subject to state and federal statutes as applicable. NOTE: this is not an Orange Book product and has not been subjected to FDA therapeutic equivalency or other equivalency testing. No representation is made as to generic status or bioequivalency. Each person recommending a prescription substitution using this product shall make such recommendations based on each such person’s professional opinion and knowledge, upon evaluating the active ingredients, excipients, inactive ingredients and chemical formulation information provided herein.
MANUFACTURED FOR
Acella Pharmaceuticals, LLC
Alpharetta, GA 30009
1-800-541-4802
Rev. 1217-01 - PRINCIPAL DISPLAY PANEL - 40 g container label
-
INGREDIENTS AND APPEARANCE
SALICYLIC ACID
salicylic acid gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42192-134 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 60 mg in 1 g Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALCOHOL (UNII: 3K9958V90M) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42192-134-40 1 in 1 CARTON 05/19/2011 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/19/2011 Labeler - Acella Pharmaceuticals, LLC (825380939) Establishment Name Address ID/FEI Business Operations Acella Pharmaceuticals, LLC 825380939 manufacture(42192-134)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
