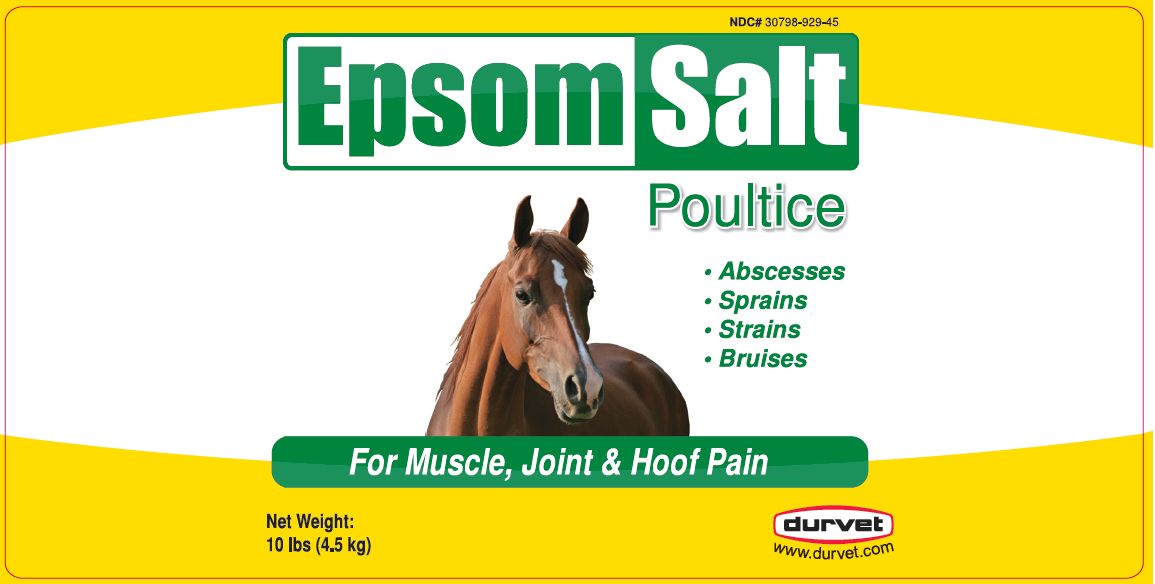
EPSOM SALT POULTICE- magnesium sulfate poultice
Epsom Salt by
Drug Labeling and Warnings
Epsom Salt by is a Animal medication manufactured, distributed, or labeled by Durvet, Inc., FIRST PRIORITY INCORPORATED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
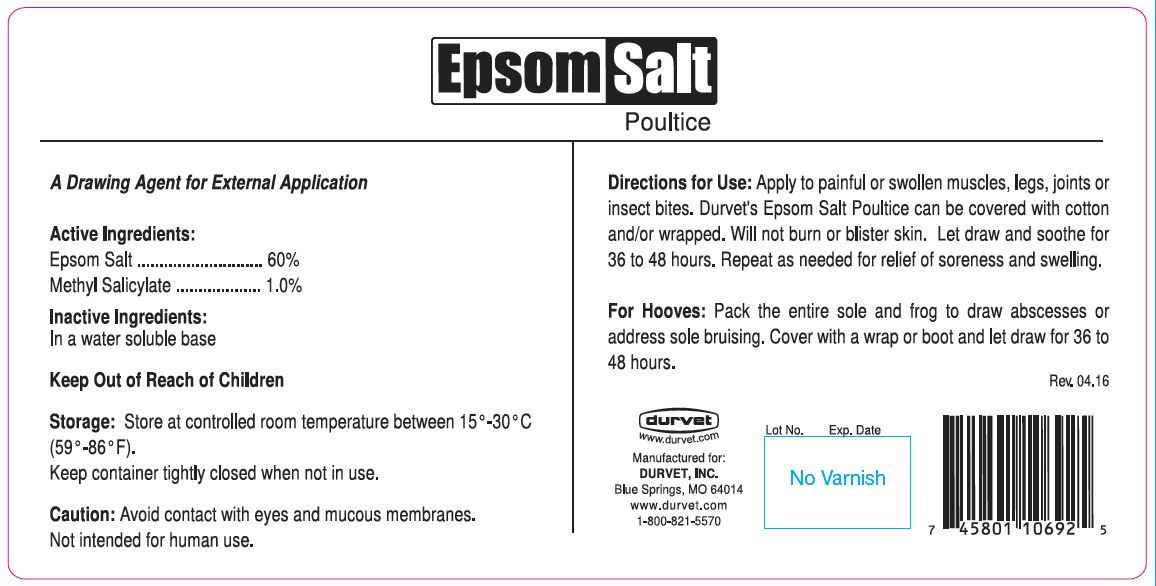
- PURPOSE
- Active Ingredients:
- Inactive Ingredients:
- KEEP OUT OF REACH OF CHILDREN
- Storage:
- Directions for Use:
- Net Contents:
- INFORMATION FOR OWNERS/CAREGIVERS
- Net Contents: 20 oz (567.0 g)
-
INGREDIENTS AND APPEARANCE
EPSOM SALT POULTICE
magnesium sulfate poulticeProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC: 30798-929 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM SULFATE HEPTAHYDRATE 600.5 mg in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 10 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 30798-929-59 12 in 1 CASE 1 567.0 g in 1 JAR 2 NDC: 30798-929-45 4536 g in 1 PAIL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/11/2016 Labeler - Durvet, Inc. (056387798) Establishment Name Address ID/FEI Business Operations FIRST PRIORITY INCORPORATED 179925722 manufacture, label
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.