PANHEMATIN- hemin powder, for solution
Panhematin by
Drug Labeling and Warnings
Panhematin by is a Prescription medication manufactured, distributed, or labeled by Recordati Rare Diseases, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PANHEMATIN safely and effectively. See full prescribing information for PANHEMATIN.
PANHEMATIN® (hemin for injection)
For intravenous infusion only.
Initial U.S. Approval: 1983INDICATIONS AND USAGE
PANHEMATIN is a hemin for injection indicated for amelioration of recurrent attacks of acute intermittent porphyria temporally related to the menstrual cycle in susceptible women, after initial carbohydrate therapy is known or suspected to be inadequate. (1)
Limitations of Use
DOSAGE AND ADMINISTRATION
For intravenous infusion only.
-
Dose (2.1)
- o 1 to 4 mg/kg/day for 3 to 14 days based on the clinical signs. The standard dose in clinical practice is 3 to 4 mg/kg/day.
- o Repeat dose in more severe cases no earlier than every 12 hours. Do not exceed 6 mg/kg in any 24 hour period.
-
Administration (2.2)
- o Use sterile 0.45 micron or smaller filter to remove any undissolved particulate matter.
- o The dose may be administered directly from the vial over a period of at least 30 minutes.
- o After the infusion, flush the vein with 100 mL of 0.9% NaCl.
DOSAGE FORMS AND STRENGTHS
PANHEMATIN is available as a sterile, lyophilized powder for reconstitution for injection. Each vial contains the equivalent of 350 mg hemin, 240 mg sodium carbonate and 335 mg of sorbitol.
When mixed as directed with Sterile Water for Injection, USP, each 48 mL provides the equivalent of approximately 336 mg hematin (7 mg/mL). (3)
CONTRAINDICATIONS
Do not use in patients with known hypersensitivity to PANHEMATIN. (4)
WARNINGS AND PRECAUTIONS
- Phlebitis is possible. Utilize a large arm vein or a central venous catheter for administration to minimize the risk of phlebitis. (5.1)
- Elevated iron and serum ferritin may occur. Monitor iron and serum ferritin in patients receiving multiple administrations of PANHEMATIN. (5.2)
- PANHEMATIN has transient and mild anticoagulant effect. Avoid concurrent anticoagulant therapy. (5.3)
- Reversible renal shutdown has been observed with an excessive hematin dose (12.2 mg/kg in a single infusion). Strictly follow recommended dosage guidelines. (5.4)
- PANHEMATIN may carry a risk of transmitting infectious agents, e.g., viruses, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. (5.5)
ADVERSE REACTIONS
Most common adverse reactions in >1% of patients are headache, pyrexia, infusion site reactions, and phlebitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Recordati Rare Diseases Inc. at 1-888-575-8344 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Avoid CYP inducing drugs such as estrogens, barbituric acid derivatives and steroid metabolites which induce δ-aminolevulinic acid synthetase 1 (ALAS1) through a feedback mechanism. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2017
-
Dose (2.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing
2.2 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Phlebitis
5.2 Iron and Serum Ferritin
5.3 Anticoagulant Effects
5.4 Renal Effects
5.5 Transmissible Infectious Agents
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
PANHEMATIN is a hemin for injection indicated for the amelioration of recurrent attacks of acute intermittent porphyria temporally related to the menstrual cycle in susceptible women, after initial carbohydrate therapy is known or suspected to be inadequate.
Limitations of Use
- Before administering PANHEMATIN, consider an appropriate period of carbohydrate loading (i.e., 400 g glucose/day for 1 to 2 days) [See Dosage and Administration (2.1)].
- Attacks of porphyria may progress to a point where irreversible neuronal damage has occurred. PANHEMATIN therapy is intended to prevent an attack from reaching the critical stage of neuronal degeneration. PANHEMATIN is not effective in repairing neuronal damage.
-
2 DOSAGE AND ADMINISTRATION
For intravenous infusion only.
2.1 Dosing
- PANHEMATIN should only be used by or in consultation with physicians experienced in the management of porphyrias.
-
Before PANHEMATIN therapy is begun, the presence of acute porphyria must be diagnosed using the following criteria:
- 1. Presence of clinical symptoms suggestive of acute porphyric attack.
- 2. Quantitative measurement of porphobilinogen (PBG) in urine. The single-void urine sample should be refrigerated or frozen without additives and shielded from light for subsequent quantitative δ-aminolevulinic acid (ALA), PBG, and total porphyrin determinations. (Note: the classical Watson-Schwartz or Hoesch tests are considered to be less reliable).
- Clinical benefit from PANHEMATIN depends on prompt administration. For mild porphyric attacks (mild pain, no vomiting, no paralysis, no hyponatremia, no seizures), a trial of glucose therapy is recommended while awaiting hemin treatment or if hemin is unavailable. For moderate to severe attacks, immediate hemin treatment is recommended. Symptoms of severe attacks are severe or prolonged pain, persistent vomiting, hyponatremia, convulsion, psychosis, and neuropathy. In addition to treatment with PANHEMATIN, consider other necessary measures such as the elimination of triggering factors.
- The dose of PANHEMATIN is 1 to 4 mg/kg/day of hematin for 3 to 14 days based on the clinical signs. The standard dose in clinical practice is 3 to 4 mg/kg/day. In more severe cases this dose may be repeated no earlier than every 12 hours. Do not exceed 6 mg/kg of hematin in any 24 hour period. After reconstitution each mL of PANHEMATIN contains the equivalent of approximately 7 mg of hematin (see dosage calculation table below).
Dosage Calculation Table
1 mg hematin equivalent = 0.14 mL PANHEMATIN
2 mg hematin equivalent = 0.28 mL PANHEMATIN
3 mg hematin equivalent = 0.42 mL PANHEMATIN
4 mg hematin equivalent = 0.56 mL PANHEMATIN
- Monitor urinary concentrations of the following compounds during PANHEMATIN therapy. Effectiveness is demonstrated by a decrease in one or more of the following compounds.
-
ALA - δ-aminolevulinic acid
PBG - porphobilinogen
Uroporphyrin
Coproporphyrin
2.2 Preparation and Administration
- Because PANHEMATIN contains no preservative and undergoes rapid chemical decomposition in solution, it must be reconstituted immediately before use.
- Reconstitute PANHEMATIN by aseptically adding 48 mL of Sterile Water for Injection, USP, to the dispensing vial. Shake the vial well for a period of 2 to 3 minutes to aid dissolution.
- PANHEMATIN may be administered directly from the vial. After the first withdrawal from the vial, discard any solution remaining.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Since reconstituted PANHEMATIN is not transparent, any undissolved particulate matter is difficult to see when inspected visually. Therefore, terminal filtration through a sterile 0.45 micron or smaller filter is recommended.
- Do not add other drug or chemical agent to a PANHEMATIN fluid admixture.
- Infuse the dose over a period of at least 30 minutes via a separate line.
- After the infusion, flush the vein with 100 mL of 0.9% NaCl.
-
3 DOSAGE FORMS AND STRENGTHS
PANHEMATIN is available as a sterile, lyophilized black powder in single dose dispensing vials. Each vial contains the equivalent of 350 mg hemin, 240 mg sodium carbonate and 335 mg of sorbitol. When mixed as directed with Sterile Water for Injection, USP, each 48 mL provides the equivalent of approximately 336 mg hematin (7 mg/mL).
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Phlebitis
A large arm vein or a central venous catheter should be utilized for the administration of PANHEMATIN to minimize the risk of phlebitis.
Since reconstituted PANHEMATIN is not transparent, any undissolved particulate matter is difficult to see when inspected visually. Therefore, terminal filtration through a sterile 0.45 micron or smaller filter is recommended. [See Dosage and Administration (2.2)]
5.2 Iron and Serum Ferritin
Because increased levels of iron and serum ferritin have been reported in post-marketing experience, physicians must monitor iron and serum ferritin in patients receiving multiple administrations of PANHEMATIN [See Adverse Reactions (6.2)]. In case of elevated iron or serum ferritin levels, consider iron chelation therapy.
5.3 Anticoagulant Effects
Because PANHEMATIN has exhibited transient, mild anticoagulant effects during clinical studies, avoid concurrent anticoagulant therapy. The extent and duration of the hypocoagulable state induced by PANHEMATIN has not been established.
5.4 Renal Effects
Recommended dosage guidelines should be strictly followed. Reversible renal shutdown has been observed in a case where an excessive hematin dose (12.2 mg/kg) was administered in a single infusion. Oliguria and increased nitrogen retention occurred although the patient remained asymptomatic. No worsening of renal function has been seen with administration of recommended dosages of hematin.
5.5 Transmissible Infectious Agents
Because PANHEMATIN is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creuzfeldt-Jacob disease (vCJD) agent, and theoretically the Creuzfeldt-Jacob disease (CJD) agent. The risk that this product may transmit an infectious agent has been reduced by screening blood donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating certain viruses. Despite these measures, this product can still potentially transmit disease. There is also the possibility that unknown infectious agents may be present in the product.
All infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Recordati Rare Diseases at 1-888-575-8344.
-
6 ADVERSE REACTIONS
The most common adverse reactions (occurring in >1% of patients) are: headache, pyrexia, infusion site reactions, and phlebitis.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of PANHEMATIN use was evaluated in a compassionate use study. A total of 130 patients were treated with hemin for acute attacks, prophylaxis or both. Of those, 111 patients were administered hemin for treatment of 305 acute porphyria attacks and to 40 patients for prophylaxis. The majority (92%) of patients were Caucasian. Most (72%) were female; all adult patients had a mean age ± SD of 40.3 ± 12.3 years. Proportionally more females (15 out of 19) received prophylaxis or a combination of acute treatment and prophylaxis (19 out of 21). For the treatment of acute attacks, patients received 2 to 4 mg/kg/day PANHEMATIN intravenously for 1 to 9 doses. For prophylaxis patients, the most common doses were weekly or biweekly infusions. Table 1 summarizes adverse reactions occurring in >1% of patients treated with PANHEMATIN, categorized by body system and order of decreasing frequency.
Table 1: Adverse Reactions in >1% of Patients Treated with PANHEMATIN System Organ Class
Preferred TermAdverse Events
N (% of Total Adverse Events)
Description
Total
Possibly or Probably Related to Treatment
Infections and infestations
Cellulitis
3 (1.5%)
2 (1.0%)
Nervous System Disorders
Headache
18 (9.2%)
5 (2.6%)
Vascular Disorders
Phlebitis / Injection site phlebitis
7 (3.6%)
6 (3.1%)
Skin and subcutaneous tissue disorders
Rash
3 (1.5%)
3 (1.5%)
General Disorders and Administration Site Conditions
Pyrexia
9 (4.6%)
6 (3.1%)
Catheter-related Complication
7 (3.6%)
3 (1.5%)
6.2 Postmarketing Experience
The following adverse reactions associated with the use of PANHEMATIN were identified in open-label clinical trials or postmarketing reports. Because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: thrombocytopenia, coagulopathy (including prolonged prothrombin time and prolonged partial thromboplastin time), and hemolysis
Immune System Disorders: hypersensitivity reactions including a report of infusion-related anaphylactoid reaction presenting as circulatory collapse
Vascular Disorders: injection site venous thrombosis including some that occurred in large veins such as venae cavae
General Disorders and Administration Site Conditions: infusion site reactions (such as erythema, pain, bleeding and extravasation)
Metabolism and Nutrition Disorders: iron overload and serum ferritin increased [See Warnings and Precautions (5.2)]
-
7 DRUG INTERACTIONS
PANHEMATIN therapy is intended to limit the rate of porphyria/heme biosynthesis possibly by inhibiting the enzyme δ-aminolevulinic acid synthetase 1 (ALAS1) [See Clinical Pharmacology (12.1)]. Most of the heme synthesized in liver is used for the production of cytochrome P450 (CYP) enzymes. Therefore, avoid CYP inducing drugs (such as estrogens, barbituric acid derivatives and steroid metabolites) while on PANHEMATIN therapy, because these drugs increase the activity of ALAS leading to induction of ALAS1 through a feedback mechanism.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
About 50% of the women with acute intermittent porphyria experience an acute attack of porphyria in pregnancy and/or the puerperium. It is most severe in early pregnancy and the puerperium, and can result in fatal outcome. Although anecdotal evidence suggests safe use of hematin during pregnancy, the available human data is not sufficient to establish the presence or absence of drug-associated risk. Animal reproduction studies have not been conducted with hematin. It is also not known whether hematin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. PANHEMATIN should be given to a pregnant woman only if clearly needed.
Avoid administering hematin in severe pre-eclampsia because of a theoretical risk of potentiation of the coagulation disorder [see Warnings and Precautions (5.3)].
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for PANHEMATIN and any potential adverse effects on the breastfed child from PANHEMATIN or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients under 16 years of age have not been established.
8.5 Geriatric Use
Clinical data for subjects aged 65 and over was not sufficient to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
Reversible renal shutdown has been observed in a case where an excessive hematin dose (12.2 mg/kg) was administered in a single infusion [see Warnings and Precautions (5.4)]. Treatment of this case consisted of ethacrynic acid and mannitol.
-
11 DESCRIPTION
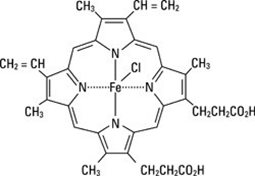
PANHEMATIN (hemin for injection) is an enzyme inhibitor derived from processed red blood cells. Hemin for injection was known previously as hematin. The term hematin has been used to describe the chemical reaction product of hemin and sodium carbonate solution. Hemin and hematin are iron containing metalloporphyrin complexes with either bound chloride or hydroxide ions, respectively. Chemically hemin is represented as chloro [7,12-diethenyl-3,8,13,17-tetramethyl- 21H,23H-porphine-2,18-dipropanoato(2-)-N21,N22,N23,N24] iron. The structural formula for hemin is:
PANHEMATIN is formatted as a sterile, lyophilized powder for intravenous administration after reconstitution. Each dispensing vial of PANHEMATIN contains the equivalent of 350 mg hemin, 240 mg sodium carbonate and 335 mg of sorbitol. The pH may have been adjusted with hydrochloric acid. When mixed as directed with Sterile Water for Injection, USP, each 48 mL provides the equivalent of approximately 336 mg hematin (7 mg/mL). The product contains no preservatives.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Heme acts to limit the hepatic and/or marrow synthesis of porphyrin. This action is likely due to the inhibition of δ-aminolevulinic acid synthetase, the enzyme which limits the rate of the porphyrin/heme biosynthetic pathway. The exact mechanism by which hematin produces symptomatic improvement in patients with acute episodes of the hepatic porphyrias has not been elucidated.
PANHEMATIN therapy for the acute porphyrias is not curative. After discontinuation of PANHEMATIN treatment, symptoms generally return although in some cases remission is prolonged. Some neurological symptoms have improved weeks to months after therapy although little or no response was noted at the time of treatment.
12.3 Pharmacokinetics
Following intravenous administration of hematin in non-jaundiced human patients, an increase in fecal urobilinogen can be observed which is roughly proportional to the amount of hematin administered. This suggests an enterohepatic pathway as at least one route of elimination. Bilirubin metabolites are also excreted in the urine following hematin injections.
Other aspects of human pharmacokinetics have not been defined.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The effectiveness of PANHEMATIN for the amelioration of recurrent attacks of acute intermittent porphyria was evaluated in five open-label studies, one compassionate-use study, case reports, and an observational study investigating patient reported outcomes in patients with acute porphyrias.
Open-Label Studies
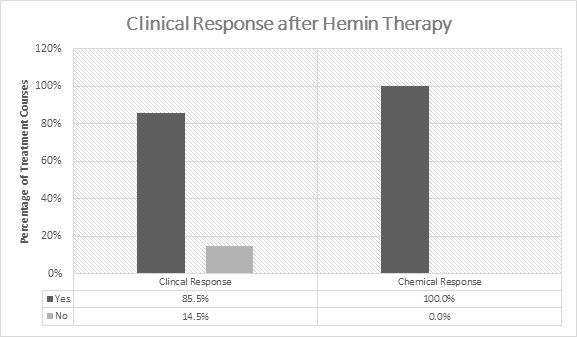
In the initial 5 open-label studies,1-5 99 patients with acute porphyrias (72 with AIP) were treated with 3-4 mg/kg/day of hemin once or twice daily. Of the 99 patients in these studies, 30 received prior or concomitant glucose administration. Patients experienced a clinical response in 85.5% (141/165) of treatment courses (Figure 1). Clinical response was defined by improvement of symptoms and reduction in pain. All patients experienced a chemical response which was defined as normalization of urinary aminolevulinic acid (ALA) and porphobilinogen (PBG).
Watson et al.1 studied the use of hemin treatment in 15 patients with acute porphyrias, of whom 11 were with AIP. Seven patients were female and four were male with an age range of 19-45 years with biochemical evidence of AIP. Preparations of 4 mg/kg IV of hemin were infused at 12- or 24-hour intervals for 1 to 4 days after trials of glucose of various durations and dosages in all patients. All patients, with exception of one, experienced a clear clinical response most of which was rapid after hemin infusion. All patients also demonstrated a chemical response based on 58%-100% reduction in urinary ALA and PBG levels.
Pierach et al.2 examined the use of 2 to 4 mg/kg of hemin IV in 57 patients with acute porphyrias, of whom 43 were with AIP. Out of 82 individual acute intermittent porphyria attacks with 476 hemin infusions (82 treatment courses) administered, a clinical response was seen in 74 (90%) acute attacks. A chemical response was seen for those patients who had elevated urinary ALA and PBG levels prior to hemin treatment.
McColl et al.3 reported the use of 4 mg/kg of hemin IV given either every 12 or 24 hours for three to five days in the treatment of 13 attacks of acute porphyria in eight patients. Seven of these 8 patients had AIP. Five patients with AIP were female and two were male with a mean age of 25 years (range 19-31 years). All patients had biochemical and clinical evidence of an attack of acute porphyria at the time of hemin administration. All patients had a chemical response of approximately 50% reduction in urinary ALA and PBG from pre-treatment values. In addition, clinical response was seen after hemin treatment in a total of 7 attacks in 5 AIP patients.
Lamon et al.4 reported on 12 patients with acute porphyrias, of whom 11 were with AIP. These AIP patients received 190 infusions of approximately 2 to 4 mg/kg of hemin IV given every 12 or 24 hours for 3 to 13 days as 20 separate courses of treatment, when high carbohydrate intake (300 g for a minimum of 72 hours) and supportive measures were unsuccessful. Urinary ALA and PBG levels were collected as well as clinical signs and symptoms of AIP recorded. Out of 20 treatment courses for acute attacks, there was a clinical response in 14. All patients had significant reductions in ALA and/or PBG levels after hemin treatment (p-value in the range from less than 0.001 to 0.05).
In another study by Lamon et al.5 seven patients with acute attacks of porphyria were administered 11 hemin courses (each course: 1 mg/kg every 24 hours for 3 to 13 days). Before and during hemin administration, patients were maintained on a 250-300 g/24H carbohydrate diet. Patients had elevated urinary ALA and PBG treatment and clinical evidence of an acute attack. Chemical response of a decrease in ALA and PBG occurred in every patient (except one PBG value in one patient) when treatment lasted 5 days or longer (p<0.001).
Figure 1: Efficacy Data on Hemin in Acute Intermittent Porphyria from 5 Open-Label Studies
Compassionate Use Study
In the compassionate use, multi-center, open-label, non-comparative study6, 130 patients were enrolled with a diagnosis of acute porphyria and were treated with hemin. The patients were administered hemin for acute attacks [N=90 (69%)], prophylaxis [N=19 (15%)], or both [N=21 (16%)]. There was a subset of patients in the “both” group (acute attacks and prophylaxis) who were treated for acute attacks prior to receiving prophylactic treatment. Seventy-two percent of the patients were female and 28% were male. Hemin was administered to 111 patients (enrolled in the “acute attack” and the “both” treatment groups) for the treatment of 305 acute attacks and to 40 patients (enrolled in the “prophylaxis” and the “both” treatment groups) for prophylactic treatment. Out of the 40 patients who received prophylaxis, 19 received prophylaxis only and 21 patients were treated for up to 3 acute attacks prior to receiving prophylactic treatment. Prophylaxis treatment varied greatly in frequency with the most common hemin regimen given once a week. Clinical response was achieved if the physician determined that the admitting symptoms were resolved, there was a clinically acceptable response, or the patient went into remission.
A physician-assessed clinical response was achieved for all acute attacks in 81 (73%) of 111 patients. Ninety-four patients (85%) of 111 had ≥1 clinical response and 17 patients (15%) of 111 had no response. Among 31 of 40 patients who received hemin prophylaxis for >1 month, 21 (68%) did not require subsequent hemin treatment for acute attacks.
Case Reports
In 234 courses, patients received hemin therapy as normally prescribed by their physicians with the majority dosed between the recommended range of 3 mg/kg/day to 4 mg/kg/day for at least one course of treatment. In these patients, hemin treatment was administered immediately in 33% of recipients, within 1 day of symptom onset in 50%, and within 3 days in 75%. These groups were not mutually exclusive. Most patients [108/111 (97.3%)] received a dose of at least 3 mg/kg/day and only 3 patients (2.7%) received a dose of hemin less than 2 mg/kg/day. There were 6patients (5.4%) who were administered doses exceeding 6 mg/kg/day for 1 or more treatment courses.
Observational Patient Reported Outcomes Study
An observational study investigated patient reported outcomes in 108 patients with acute porphyrias.7 Out of 108 patients, 90 patients were with AIP and reported the following:
- 55% percent reported having received hemin during acute attacks, and 74% of these patients assessed PANHEMATIN therapy as very successful in the treatment of abdominal pain and other symptoms.
- 50% reported having received treatment with opiates during an acute attack, and 44% of these patients reported that opiates were effective.
Hemin therapy effectiveness was assessed along with glucose infusions, high carbohydrate diets, and pain medications on a scale from zero being least effective to 10 highly effective. Hemin infusions received a 7.9, glucose infusions a 4.4 (p=0.0781), high carbohydrate diets a 4.7 (p=0.0021), and pain medications a 4.2 (p=0.0049).
-
15 REFERENCES
- 1. Watson, CJ, et al., Use of Hematin in the Acute Attack of the “Inducible” Hepatic Porphyrias, Adv Intern Med. 1978;23:265-286.
- 2. Pierach CA, Bossenmaier I, Cardinal R, Weimer M, Watson CJ. Hematin therapy in porphyric attacks. Klinische Wochenschrift. 1980;58(16):829-832.
- 3. McColl KE, Moore MR, Thompson GG, Goldberg A. Treatment with haematin in acute hepatic porphyria. The Quarterly journal of medicine. 1981;50(198):161-174.
- 4. Lamon, JM, Hematin Therapy for Acute Porphyria, Medicine. 1979;58(3):252-269.
- 5. Lamon JM. Hematin Therapy in Acute Porphyria. Clin Res. 1977;25(3):471A.
- 6. Anderson, KE and Collins, S, Open-Label Study of Hemin for Acute Porphyria: Clinical Practice Implications. American Journal of Medicine. 2006;119(9):801.
- 7. Bonkovsky HL, Maddukuri VC, Yazici C, Anderson KE, Bissell M, et al. Acute porphyrias in the USA: features of 108 subjects from porphyrias consortium. Am J Med. 2014;127(12):1233-1241.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
PANHEMATIN is supplied as a sterile, lyophilized black powder in single dose dispensing vials (NDC: 55292-702-54) in a carton (NDC: 55292-702-55).
The vial stopper contains natural rubber latex.
Store lyophilized powder at 20-25°C (68-77°F).
-
17 PATIENT COUNSELING INFORMATION
- Advise the patient not to take drugs such as estrogens (e.g., oral contraceptives), barbiturates (drugs which help them to sleep and drugs sometimes used to treat epilepsy) or steroids (body hormone-like drugs), because this can trigger an attack or make the attack worse.
- Advise a female patient to inform the prescriber if she is pregnant or planning to become pregnant.
Manufactured by:
Xellia Pharmaceuticals USA, LLC
Raleigh, NC 27616For:
Recordati Rare Diseases Inc.
Lebanon, NJ 08833, U.S.A.U.S. Lic. No. 1899
RECORDATI RARE DISEASES GROUP
Panhematin® is a registered trademark of Recordati Rare Diseases Inc.
750-09241
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 55292-702-54
Single Dose VialHemin For Injection
Panhematin®350 mg Hemin per Vial
For Intravenous Infusion Only
Sterile Powder for InjectionRECORDATI RARE DISEASES GROUP
Rx only
NDC: 55292-702-55
Contains One VialHemin For Injection
Panhematin®350 mg Hemin per Vial
For Intravenous Infusion Only
Sterile Powder for InjectionRECORDATI RARE DISEASES GROUP
Rx only. -
INGREDIENTS AND APPEARANCE
PANHEMATIN
hemin powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55292-702 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Hemin (UNII: 743LRP9S7N) (Hemin - UNII:743LRP9S7N) Hemin 7 mg in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Carbonate (UNII: 45P3261C7T) Sorbitol (UNII: 506T60A25R) Hydrochloric Acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55292-702-55 1 in 1 CARTON 07/20/1983 1 NDC: 55292-702-54 48 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101246 07/20/1983 Labeler - RECORDATI RARE DISEASES, INC. (181699406) Establishment Name Address ID/FEI Business Operations Xellia Pharmaceuticals USA, LLC 079459964 MANUFACTURE(55292-702)
Trademark Results [Panhematin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PANHEMATIN 73451832 1317567 Live/Registered |
Abbott Laboratories 1983-11-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.