INFED- iron dextran injection
INFED by
Drug Labeling and Warnings
INFED by is a Prescription medication manufactured, distributed, or labeled by Henry Schein, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use INFeD safely and effectively. See full prescribing information for INFeD.INFeD® (iron dextran injection), for intravenous or intramuscular use
Initial U.S. Approval: 1974INDICATIONS AND USAGE
INFeD, an iron replacement product, is indicated for treatment of adult and pediatric patients of age 4 months and older with documented iron deficiency who have intolerance to oral iron or an unsatisfactory response to oral iron. (1) (2)
DOSAGE AND ADMINISTRATION
See Full Prescribing Information for weight-based dosing and administration information. (2) (3)
DOSAGE FORMS AND STRENGTHS
Injection: 100 mg/2 mL (50 mg/mL) in single-dose vials (3) (4)
CONTRAINDICATIONS
Known hypersensitivity to INFeD (4) (5)
WARNINGS AND PRECAUTIONS
Delayed Reactions: May occur with large intravenous doses. (5.2)
Increased Risk of Toxicity in Patients with Underlying Conditions: Monitor for toxicity in these patients. (5.3)
Iron Overload: Excessive therapy can lead to iatrogenic hemosiderosis. Do not administer to patients with iron overload. Periodically monitor hematologic and iron parameters. (5.4) (6)ADVERSE REACTIONS
USE IN SPECIFIC POPULATIONS
Revised: 11/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
BOXED WARNING
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
13 NONCLINICAL TOXICOLOGY
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
These highlights do not include all the information needed to use INFeD safely and effectively. See full prescribing information for INFeD.
INFeD® (iron dextran injection), for intravenous or intramuscular use
Initial U.S. Approval: 1974WARNING: RISK FOR ANAPHYLACTIC-TYPE REACTIONS
SEE FULL PRESCRIBING INFORMATION FOR COMPLETE BOXED WARNING.
Anaphylactic-type reactions, including fatalities, have been reported following the parenteral administration of iron dextran injection. (5.1)
Have resuscitation equipment and personnel trained in the detection and treatment of anaphylactic-type reactions readily available during INFeD administration.
Administer a test INFeD dose prior to the first therapeutic dose. If no signs or symptoms of anaphylactic-type reactions follow the test dose, administer the full therapeutic INFeD dose.
During all INFeD administrations, observe for signs or symptoms of anaphylactic-type reactions. Fatal reactions have been reported following the test dose of iron dextran injection. Fatal reactions have also occurred in situations where the test dose was tolerated.
Use INFeD only in patients in whom clinical and laboratory investigations have established an iron deficient state not amenable to oral iron therapy.
Patients with a history of drug allergy or multiple drug allergies may be at increased risk of anaphylactic-type reactions to INFeD. (5.3) - 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Pre-Treatment Information
Discontinue administration of any iron-containing products prior to administration of INFeD.Assess baseline hematologic (hemoglobin and hematocrit) and iron storage parameters (serum iron, total iron binding capacity, and percent saturation of transferrin) to monitor response to therapy.
Administer a test dose of INFeD prior to administration of therapeutic dose [see Dosage and Administration (2.4)].
2.2 Recommended Dosage for Iron Deficiency Anemia
Calculate the INFeD dose based upon Table 1 and formulas below. Continue INFeD until hemoglobin is within the normal range and iron stores are replete.Administer daily doses of no more than 2 mL of INFeD until the total required dose is administered. Monitor response to therapy by evaluating hematologic parameters (hemoglobin and hematocrit) and iron storage parameters (serum iron, total iron binding capacity, and percent saturation of transferrin). Iron storage parameters may improve prior to hematologic parameters. Serum ferritin may not be an accurate measure of body iron stores in patients on chronic dialysis.
Table 1: Total INFeD Requirement for Hemoglobin Restoration and Iron Stores Replacement in Patients with Iron Deficiency Anemia*
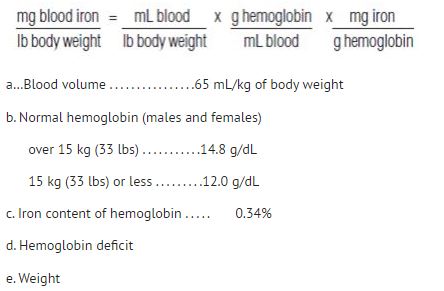
Alternatively, the total dose may be calculated using the formulas below:
Adults and Children over 15 kg (33 lbs)
Dose (mL) = 0.0442 (Desired Hb - Observed Hb) x LBW + (0.26 x LBW)
Based on:
Desired Hb = the target hemoglobin in g/dL [Normal hemoglobin (males and females) for body weight over 15 kg (33 lbs) is 14.8 g/dL.]
Observed Hb = the patient’s current hemoglobin in g/dL
LBW = Lean body weight in kg [A patient’s lean body weight (or actual body weight if less than lean body weight) should be utilized when determining dosage.]
For males: LBW = 50 kg + 2.3 kg for each inch of patient’s height over 5 feet
For females: LBW = 45.5 kg + 2.3 kg for each inch of patient’s height over 5 feet
To calculate a patient's weight in kg when lbs are known:Weight in Kg
Children 5 to 15 kg (11 to 33 lbs)
Otherwise, the total dose may be calculated using the formula below:
Dose (mL) = 0.0442 (Desired Hb - Observed Hb) x W + (0.26 x W)
Based on:
Desired Hb = the target hemoglobin in g/dL [Normal hemoglobin for children with body weight of 15 kg (33 lbs) or less is 12 g/dL.]
W = body weight in kg
To calculate a patient's weight in kg when lbs are known:Weight in Kg
2.3 Recommended Dosage of Iron Replacement for Blood Loss
Calculate the INFeD dose based upon the formula below which is based upon the approximate amount of blood loss and pretreatment hematocrit.The formula is based on the approximation that 1 mL of normocytic, normochromic red cells contains 1 mg of elemental iron.
INFeD Dose (in mL) = [Blood loss (in mL) x hematocrit] ÷ 50 mg/mL
Example: Blood loss of 500 mL with 20% hematocrit
Replacement Iron = 500 x 0.20 = 100 mg
INFeD dose volume = INFeD dose volume
2.4 Administration
The total volume of INFeD required for the treatment of iron deficiency anemia is determined from Table 1 or the appropriate formula listed [see Dosage and Administration (2.2)].The total volume of INFeD required for the treatment of iron replacement for blood loss is determined from an appropriate formula listed [see Dosage and Administration (2.3)].
NOTE: Do not mix INFeD with other medications or add to parenteral nutrition solutions for intravenous infusion.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever the solution and container permit.
Discard unused portion.
Intravenous Injection
Test Dose
Prior to the first intravenous INFeD therapeutic dose, administer an intravenous test dose of 0.5 mL [see BOXED WARNING and Warnings and Precautions (5.1)]. Administer the test dose at a gradual rate over at least 30 seconds. Delay administration of the initial therapeutic INFeD dose until 1 hour or more after the test dose. If a hypersensitivity reaction occurs with the test dose, manage medically and do not administer further doses of INFeD.
INFeD is given undiluted at a slow gradual rate not to exceed 50 mg (1 mL) per minute.
The maximum daily dose of INFeD should not exceed 2 mL.
Intramuscular Injection
Test Dose
Prior to the first intramuscular INFeD therapeutic dose, administer an intramuscular test dose of 0.5 mL [see BOXED WARNING and Warnings and Precautions (5.1)]. Administer the test dose at a gradual rate over at least 30 seconds into the buttock. Delay administration of the initial therapeutic INFeD dose until 1 hour or more after the test dose. If a hypersensitivity reaction occurs with the test dose, manage medically and do not administer further doses of INFeD.
If no adverse reactions are observed, INFeD can be given according to the following schedule until the calculated total required dose has been reached. Each day’s dose should not exceed 0.5 mL (25 mg of iron) for infants with body weight under 5 kg (11 lbs); 1 mL (50 mg of iron) for children with body weight under 10 kg (22 lbs); and 2 mL (100 mg of iron) for other patients.
The maximum daily dose of INFeD should not exceed 2 mL.
INFeD should be injected only into the muscle mass of the upper outer quadrant of the buttock - never into the arm or other exposed areas - and should be injected deeply, with a 2-inch or 3-inch 19 or 20 gauge needle. If the patient is standing, he/she should be bearing his/her weight on the leg opposite the injection site, or if in bed, he/she should be in the lateral position with injection site uppermost. To avoid injection or leakage into the subcutaneous tissue, a Z-track technique (displacement of the skin laterally prior to injection) is recommended.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylactic-type reactions, some of which have been life-threatening and fatal, have been reported following the parenteral administration of iron dextran products, including INFeD. Such reactions have been generally characterized by sudden onset of respiratory difficulty and/or cardiovascular collapse. Fatal reactions have been reported following the test dose of iron dextran and have also occurred in situations where the test dose was tolerated.Administer only in a setting where resuscitation equipment and medications are available. Administer a test dose of INFeD prior to the first therapeutic dose [see Dosage and Administration (2.4)]. Observe patients for at least one hour after the test dose before administering the remainder of the initial therapeutic dose. During all INFeD administrations, observe patients for signs or symptoms of anaphylactic-type reactions. Use INFeD only in patients in whom clinical and laboratory investigations have established an iron deficient state not amenable to oral iron therapy.
The factors that affect the risk for anaphylactic-type reactions to iron dextran products are not fully known but limited clinical data suggest the risk may be increased among patients with a history of drug allergy or multiple drug allergies. Additionally, concomitant use of angiotensin-converting enzyme inhibitor drugs may increase the risk for reactions to an iron dextran product. The extent of risk for anaphylactic-type reactions following exposure to any specific iron dextran product is unknown and may vary among the products.
If hypersensitivity reactions occur during administration, stop INFeD immediately and manage reaction medically.
5.2 Delayed Reactions
Large intravenous doses, such as used with total dose infusions (TDI), have been associated with an increased incidence of adverse reactions. The adverse reactions are frequently delayed (1 to 2 days) reactions typified by one or more of the following symptoms: arthralgia, backache, chills, dizziness, moderate to high fever, headache, malaise, myalgia, nausea, and vomiting. The onset is usually 24 to 48 hours after administration and symptoms generally subside within 3 to 4 days. The etiology of these reactions is not known. Do not exceed a total daily dose of 2 mL undiluted INFeD.5.3 Increased Risk of Toxicity in Patients with Underlying Conditions
Monitor for iron toxicity when INFeD is used in patients with serious impairment of liver function. It should not be used during the acute phase of infectious kidney disease.Adverse reactions experienced following administration of INFeD may exacerbate cardiovascular complications in patients with pre-existing cardiovascular disease.
Patients with rheumatoid arthritis may have an acute exacerbation of joint pain and swelling following the administration of INFeD.
Patients with a history of significant allergies and/or asthma may have an increased risk of hypersensitivity reactions [see Dosage and Administration (5.1)].
5.4 Iron Overload
Excessive therapy with parenteral iron can lead to excess storage of iron with the possibility of iatrogenic hemosiderosis. All adult and pediatric patients receiving INFeD require periodic monitoring of hematologic and iron parameters (hemoglobin, hematocrit, serum ferritin and transferrin saturation). Do not administer INFeD to patients with evidence of iron overload. -
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
Delayed Reactions [see Warnings and Precautions (5.2)]
Increased Risk of Toxicity in Patients with Underlying Conditions [see Warnings and Precautions (5.3)]
Iron Overload [see Warnings and Precautions (5.4)]
Fetal bradycardia [see Use in Specific Populations (8.1)]
The following adverse reactions associated with the use of INFeD were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.Blood and lymphatic system disorders: Leukocytosis, lymphadenopathy.
Cardiac disorders: Cardiac arrest, tachycardia, bradycardia, arrhythmias.
Gastrointestinal disorders: Abdominal pain, nausea, vomiting, diarrhea.
General disorders and administration site conditions: chest pain, chest tightness, weakness, malaise, febrile episodes, chills, shivering, sterile abscess, atrophy/fibrosis (intramuscular injection site), brown skin and/or underlying tissue discoloration (staining), soreness or pain at or near intramuscular injection sites, swelling, inflammation.
Musculoskeletal and connective tissue disorders: Arthralgia, arthritis (may represent reactivation in patients with quiescent rheumatoid arthritis – [see Warnings and Precautions (5.3)], myalgia, backache.
Nervous system disorders: Convulsions, seizures, syncope, headache, unresponsiveness, paresthesia, dizziness, numbness, unconsciousness, altered taste.
Psychiatric disorders: Disorientation
Respiratory, thoracic and mediastinal disorders: Respiratory arrest, dyspnea, bronchospasm, wheezing.
Renal and urinary disorders: Hematuria.
Skin and subcutaneous disorders: Urticaria, pruritus, purpura, rash, sweating.
Vascular disorders: Cyanosis, shock, hypertension, hypotension, flushing (flushing and hypotension may occur from too rapid injections by the intravenous route), local phlebitis at or near intravenous injection site.
-
7 DRUG INTERACTIONS
7.1 Drug/Laboratory Test Interactions
Drug interactions involving INFeD have not been studied.Concomitant use of angiotensin-converting enzyme inhibitor drugs may increase the risk for anaphylactic-type reactions to an iron dextran product.
Large doses of iron dextran (5 mL or more) have been reported to give a brown color to serum from a blood sample drawn 4 hours after administration.
INFeD may cause falsely elevated values of serum bilirubin and falsely decreased values of serum calcium.
Serum iron determinations (especially by colorimetric assays) may not be meaningful for 3 weeks following the administration of INFeD.
Examination of the bone marrow for iron stores may not be meaningful for prolonged periods following iron dextran therapy because residual iron dextran may remain in the reticuloendothelial cells.
Bone scans involving 99m Tc-diphosphonate have been reported to show a dense, crescentic area of activity in the buttocks, following the contour of the iliac crest, 1 to 6 days after intramuscular injections of INFeD.
Bone scans with 99m Tc-labeled bone seeking agents, in the presence of high serum ferritin levels or following INFeD infusions, have been reported to show reduction of bony uptake, marked renal activity, and excessive blood pool and soft tissue accumulation.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Parenteral iron administration may be associated with hypersensitivity reactions [see Warnings and Precautions (5.1)], which may have serious consequences, such as fetal bradycardia (see Clinical Considerations). Advise pregnant persons of the potential risk to the fetus. Available data from postmarketing reports with iron dextran use in pregnancy are insufficient to assess the risk of major birth defects or miscarriage. There are risks to the pregnant person and fetus associated with untreated iron deficiency anemia in pregnancy (see Clinical Considerations). Iron dextran has been shown to be teratogenic and embryocidal in mice, rats, rabbits, dogs, and monkeys when given in doses of about 3 times the maximum human dose.The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Untreated iron deficiency anemia (IDA) in pregnancy is associated with adverse maternal outcomes such as post-partum anemia. Adverse pregnancy outcomes associated with IDA include increased risk for preterm delivery and low birth weight.Fetal/Neonatal Adverse Reactions
Severe adverse reactions including circulatory failure (severe hypotension, shock including in the context of anaphylactic reaction) may occur in pregnant persons with intravenous iron administration (such as INFeD) which may have serious consequences on the fetus such as fetal bradycardia, especially during the second and third trimester.Data
Animal Data
No consistent adverse fetal effects were observed in mice, rats, rabbits, dogs, and monkeys at doses of 50 mg iron/kg or less. Fetal and maternal toxicity has been reported in monkeys at a total intravenous dose of 90 mg iron/kg over a 14 day period. Similar effects were observed in mice and rats on administration of a single dose of 125 mg iron/kg. Fetal abnormalities in rats and dogs were observed at doses of 250 mg iron/kg and higher. The animals used in these tests were not iron deficient.8.2 Lactation
Risk Summary
Trace amounts of unmetabolized iron dextran are present in human milk. There are no data on the effects of iron dextran in breastfed infants or effects on milk production. The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for INFeD in addition to any potential adverse effects on the breastfed child from the drug or from the underlying maternal condition.8.4 Pediatric Use
INFeD is not recommended for use in infants under 4 months of age [see Dosage and Administration (2.2)].Reports in the literature from countries outside the United States (in particular, New Zealand) have suggested that the use of intramuscular iron dextran in neonates has been associated with an increased incidence of gram-negative sepsis, primarily due to E. Coli.
- 10 OVERDOSAGE
-
11 DESCRIPTION
INFeD (iron dextran injection USP) is an iron replacement product provided as a dark brown, slightly viscous sterile liquid complex of ferric hydroxide and dextran for intravenous or intramuscular use.
Each mL contains the equivalent of 50 mg of elemental iron (as an iron dextran complex), approximately 0.9% sodium chloride, in water for injection. Sodium hydroxide and/or hydrochloric acid may have been used to adjust pH. The pH of the solution is between 4.5 to 7.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The circulating iron released from iron dextran, which is subject to physiological control, replenishes hemoglobin and depleted iron stores.12.2 Pharmacodynamics
Changes in serum ferritin levels represent the changes in calculated cellular non-heme iron levels. After administration of iron dextran, evidence of a therapeutic response can be seen as an increase in the reticulocyte count.12.3 Pharmacokinetics
Following intramuscular injection, INFeD is absorbed within 72 hours with any remaining iron absorbed over the ensuing 3 to 4 weeks. Various studies involving intravenously administered 59Fe iron dextran to iron deficient subjects, some of whom had coexisting disease, have yielded half-life values ranging from 5 hours to more than 20 hours. The 5 hour value was determined for 59Fe iron dextran from a study that used laboratory methods to separate the circulating 59Fe iron dextran from the transferrin bound 59Fe. The 20 hour value reflects a half-life determined by measuring total 59Fe, both circulating and bound. It should be understood that these half-life values do not represent clearance of iron from the body. Serum ferritin peaks approximately 7 to 9 days after an intravenous dose of INFeD and returns to baseline after about 3 weeks.Absorption
Following intramuscular administration, INFeD is absorbed from the injection site into the capillaries and the lymphatic system.Distribution
The circulating iron is bound to the available protein moieties to form hemosiderin or ferritin, or to a lesser extent to transferrin.Elimination
The half-life of free iron in the plasma circulation is approximately 5 hours. The half-life of total iron, including both circulating and bound, is approximately 20 hours. These half-life values do not represent clearance of iron from the body.Metabolism
Following administration of INFeD, circulating iron dextran is split by the cells of the reticuloendothelial system into its components of iron and dextran.Excretion
Negligible amounts of iron are lost via the urinary or alimentary pathways after administration of iron dextran. Dextran, a polyglucose, is either metabolized or excreted.Specific Populations
Patients with Renal Impairment
In vitro studies have shown that removal of iron dextran by dialysis is negligible. Six different dialyzer membranes were investigated (polysulfone, cuprophane, cellulose acetate, cellulose triacetate, polymethylmethacrylate and polyacrylonitrile), including those considered high efficiency and high flux. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
The intramuscular injection of iron-carbohydrate complexes may be associated with an increased risk of carcinogenesis. In mice, rats, rabbits, and possibly hamsters, it has been demonstrated that these complexes may produce sarcoma following repeated administration of large or small doses of iron-carbohydrate complexes at a single injection site. There have been several reports in the literature describing tumors at the injection site in humans who had previously received intramuscular injections of iron-carbohydrate complexes. -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
INFeD (iron dextran injection USP) containing 50 mg of elemental iron per mL, is available as a dark brown, slightly viscous, sterile solution in 2 mL single-dose amber vials in cartons of 10 (NDC: 0023-6082-10).Product repackaged by: Henry Schein, Inc., Bastian, VA 24314 From Original Manufacturer/Distributor's NDC and Unit of Sale To Henry Schein Repackaged Product NDC and Unit of Sale Total Strength/Total Volume (Concentration) per unit NDC: 0023-6082-10
2 mL single dose amber vial in cartons of 10NDC: 0404-9879-02
1 2mL single dose vial in a bag
(Vial bears NDC: 0023-6082-10)50 mg of elemental iron per mL 16.2 Stability and Storage
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature]. -
17 PATIENT COUNSELING
Hypersensitivity Reactions
Question patients regarding any prior history of reactions to parenteral iron products. Advise patients to immediately report any symptoms of hypersensitivity that develop during and following INFeD administration such as arthralgia, backache, chills, dizziness, moderate to high fever, headache, malaise, myalgia, nausea, and vomiting [see Warnings and Precautions (5.1)].Delayed Reactions
Advise patients that delayed reactions can occur and that these must be reported to their healthcare provider immediately [see Warnings and Precautions (5.2)].Increased Risk of Toxicity in Patients with Underlying Conditions
Advise patients to inform their healthcare provider if any liver impairment is identified as this may cause iron toxicity. Advise patients to consult their healthcare provider should they start to show symptoms of acute kidney infection as INFeD should not be used [see Warnings and Precautions (5.3)].Advise patients with pre-existing cardiovascular disease and rheumatoid arthritis that INFeD administration may exacerbate symptoms and to contact their healthcare provider if any symptoms occur [see Warnings and Precautions (5.3)].
Advise patients with history of significant allergies and/or asthma to inform their healthcare provider as the risk of hypersensitivity reactions may be increased [see Warnings and Precautions (5.3)].
Iron Overload
Advise the patient to consult a healthcare provider before taking any other iron containing products as this may cause serious side effects [see Warnings and Precautions (5.4)].Pregnancy
Advise pregnant persons about the risk of hypersensitivity reactions which may have serious consequences for the fetus [see Use in Specific Populations (8.1)].For all medical inquiries contact:
Allergan
Medical Communications
1-800-678-1605Manufactured By:
Patheon Italia S.p.A.
Ferentino, Italy 03013Distributed By:
Allergan USA, Inc.
Madison, NJ 07940© 2021 Allergan. All rights reserved.
INFeD® is a registered trademark of Allergan Sales, LLC.v5.0USPI6082
- Sample Package Label
-
INGREDIENTS AND APPEARANCE
INFED
iron dextran injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0404-9879(NDC:0023-6082) Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IRON DEXTRAN (UNII: 95HR524N2M) (FERRIC CATION - UNII:91O4LML611) FERRIC CATION 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0404-9879-02 1 in 1 BAG 01/11/2022 1 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017441 01/11/2022 Labeler - Henry Schein, Inc. (012430880)
Trademark Results [INFED]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 INFED 74185675 1717425 Live/Registered |
ALLERGAN SALES, LLC 1991-07-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
