HEADACHE RELIEF EXTRA STRENGTH- acetaminophen, aspirin and caffeine tablet, film coated
Headache Relief by
Drug Labeling and Warnings
Headache Relief by is a Otc medication manufactured, distributed, or labeled by Valu Merchandisers Company, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients (in each tablet)
- Purpose
- Uses
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include:
- shock
- facial swelling
- hives
- asthma (wheezing)
Allergy alert: Acetaminophen may cause severe skin reactions.
Symptoms may include:- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg of acetaminophen in 24 hours
- 3 or more alcoholic drinks every day while using this product
- with other drugs containing acetaminophen
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heartbeat.
Do not use
- if you have ever had an allergic reaction to acetaminophen, aspirin, or any other pain reliever/fever reducer
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if
- you have liver disease
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis or kidney disease
- you have asthma
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
- taking a prescription drug for diabetes, gout, or arthritis
- taking any other drug or are under a doctor's care for any serious condition
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away.
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- ringing in the ears or a loss of hearing occurs
- pain gets worse or lasts for more than 10 days
- fever gets worse or lasts for more than 3 days
- redness or swelling is present
- new symptoms appear
- shock
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
COMPARE TO ACTIVE INGREDIENTS OF EXCEDRIN® EXTRA STRENGTH†
Best Choice®
EXTRA STRENGTH
Headache ReliefACETAMINOPHEN,
ASPIRIN (NSAID) AND CAFFEINE
Pain Reliever / Pain Reliever Aid
ANALGESIC100 TABLETS Actual Size
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
†This product is not manufactured or distributed by Novartis AG, owner of the registered trademark Excedrin® Extra Strength.
50844 REV0418F15912Best Choice®
100% Guaranteed
www.bestchoicebrand.comPROUDLY DISTRIBUTED BY:
VALU MERCHANDISERS, CO.
5000 KANSAS AVE
KANSAS CITY, KS 66106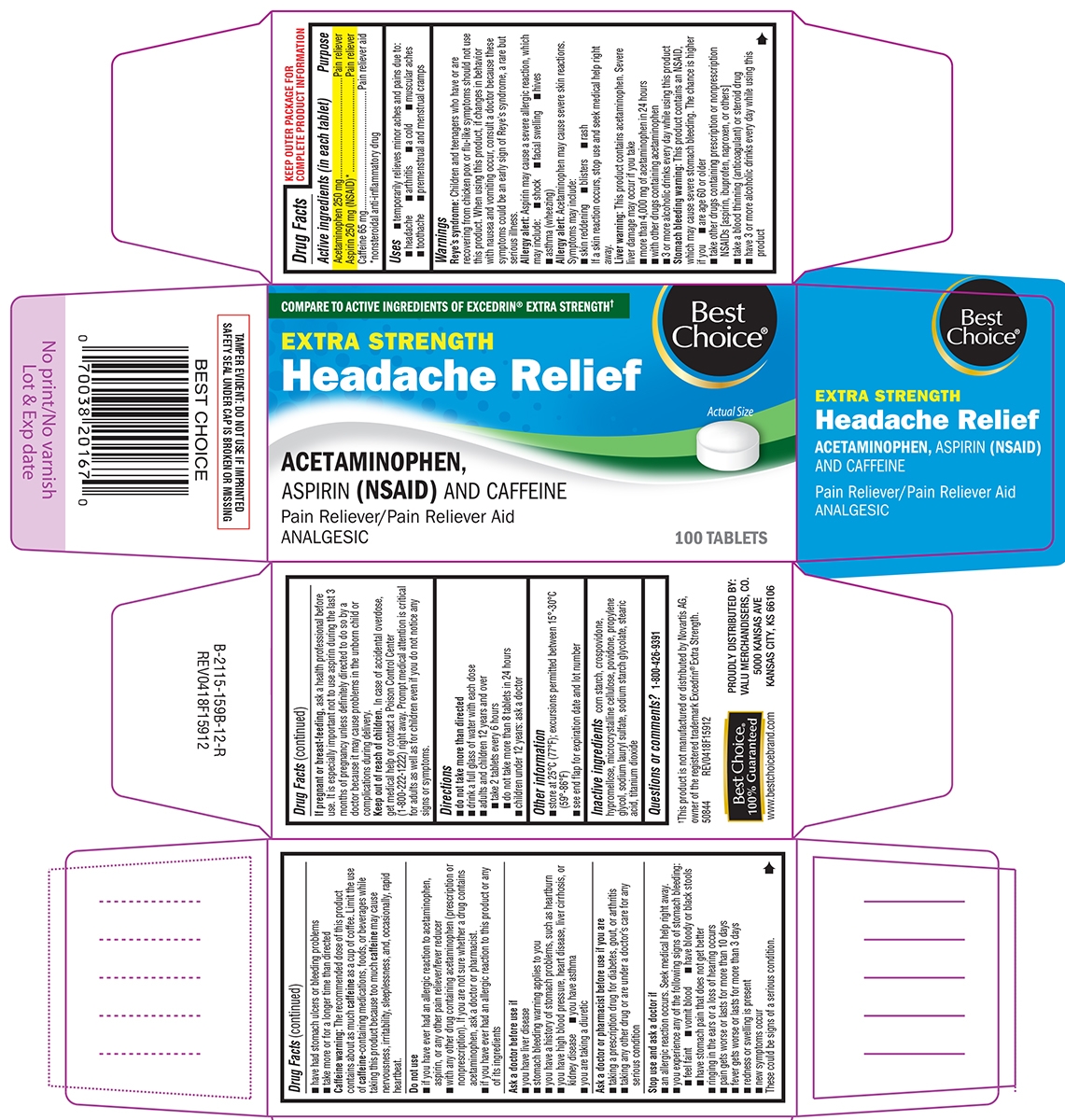
Best Choice 44-159B
-
INGREDIENTS AND APPEARANCE
HEADACHE RELIEF EXTRA STRENGTH
acetaminophen, aspirin and caffeine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63941-159 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 250 mg ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 250 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 65 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) STARCH, CORN (UNII: O8232NY3SJ) POVIDONE (UNII: FZ989GH94E) CROSPOVIDONE (UNII: 2S7830E561) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color WHITE Score no score Shape ROUND Size 11mm Flavor Imprint Code 44;159 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63941-159-12 1 in 1 CARTON 11/17/1992 1 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part343 11/17/1992 Labeler - Valu Merchandisers Company (868703513) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(63941-159) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(63941-159) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(63941-159) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(63941-159)
Trademark Results [Headache Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HEADACHE RELIEF 78847467 not registered Dead/Abandoned |
Gary A. L'Europa 2006-03-28 |
 HEADACHE RELIEF 76413753 not registered Dead/Abandoned |
ISI Brands, Inc. 2002-05-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.