ZO MEDICAL MELAMIN-C SKIN BLEACHING AND CORRECTING WITH VITAMIN C HYDROQUINONE- hydroquinone cream
ZO MEDICAL MELAMIN-C Skin Bleaching and Correcting with Vitamin C Hydroquinone by
Drug Labeling and Warnings
ZO MEDICAL MELAMIN-C Skin Bleaching and Correcting with Vitamin C Hydroquinone by is a Prescription medication manufactured, distributed, or labeled by ZO Skin Health, Inc., G.S. COSMECEUTICAL USA, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Hydroquinone is 1, 4-benzendiol, with a chemical formula of C6H6O2 and a molecular weight of 110.11.
The structural formula is:
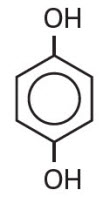
C6H6O2
Each gram of MELAMIN-C™ (Hydroquinone USP, 4%) Skin Bleaching & Correcting Crème with 20% Vitamin C contains Hydroquinone USP 40mg/gm in a base of ascorbic acid, ascorbyl palmitate, caprylic/capric triglyceride, caprylyl methicone, cetyl PEG/PPG-10/1 dimethicone, cyclotetrasiloxane, dimethicone, dimethicone crosspolymer, tocopheryl acetate, ethoxydiglycol, helianthus annuus (sunflower) seed oil, oleyl alcohol, pluenetia volubilis seed oil, retinyl palmitate, rosmarinus officinalis (rosemary) leaf extract, squalene, tocopherol, zanthoxylum bungeanum fruit extract.
-
CLINICAL PHARMACOLOGY
Topical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3,4-dihydroxyphenylalanine (DOPA) and suppression of other melanocyte metabolic processes. Exposure to sunlight or ultraviolet light will cause re-pigmentation of bleached areas, which may be prevented by the use of the sunscreen agents.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Hydroquinone is a skin bleaching agent which may produce undesired effects if not used as directed. The physician should be familiar with the contents of this insert before prescribing or dispensing this product.
Avoid unnecessary sun exposure, use an effective broad-spectrum sunscreen agent or protective clothing should be worn to cover bleached skin to prevent re-pigmentation from occurring.
Hydroquinone may produce exogenous ochronosis, a gradual blue-black darkening of the skin. If his condition occurs, discontinue treatment and consult your physician.
Avoid contact with eyes and mucous membranes. Keep out of reach of children. In case of accidental ingestion, call a physician or a poison control center immediately.
-
PRECAUTIONS
Test for skin sensitivity before using by applying a small amount to an unbroken patch of skin; check within 24 hours. Minor redness is not a contraindication, but where there is itching or vesicle formation or excessive inflammatory response, further treatment is not advised. Close patient supervision is recommended.
Drug Interactions
Patients are cautioned on concomitant use of medications that are known to be photosensitizing.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies of hydroquinone in animals have demonstrated some evidence of carcinogenicity. The carcinogenic potential of hydroquinone in humans is unknown.
Pregnancy Category C
Animal reproduction studies have not been conducted with topical hydroquinone. It is also not known whether topical hydroquinone can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Topical hydroquinone should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when topical hydroquinone is administered to a nursing woman.
Pediatric Use
Safety and effectiveness for pediatric patients below the age of 12 years have not been established.
Adverse Reactions
The following reactions have been reported: dryness and fissuring of paranasal and infraorbital areas, erythema, and stinging. Occasional hypersensitivity (localized contact dermatitis) may develop. If this occurs, the medication should be discontinued, and the physician notified immediately.
Overdosage
There have been no system reactions reported from the use of topical hydroquinone. However, treatment should be limited to relatively small areas of the body at one time, since some patients experience a transient skin reddening and a mild burning sensation which does not preclude treatment.
-
DRUG DOSAGE AND ADMINISTRATION
A thin layer of MELAMIN-C™ (Hydroquinone USP, 4%) Skin Bleaching & Correcting Crème with 20% vitamin C should be applied to the affected area twice daily or as directed by a physician. If no improvement is seen after 8-12 weeks of treatment, use of this product should be discontinued. There is no recommended dosage for pediatric patients under 12 years of age except under the advice and supervision of a physician.
-
HOW SUPPLIED
MELAMIN-C™ (Hydroquinone USP, 4%) Skin Bleaching & Correcting Crème with 20% Vitamin C is available as follows:
2.99 Oz. (85 g) Bottle / NDC: 42851-034-85
1.19 Oz. (34 g) Bottle / NDC: 42851-034-34
-
PRINCIPAL DISPLAY PANEL - 85 g Bottle Carton
ZO®MEDICAL
BY ZEIN OBAGI MDNDC: 42851-034-85
MELAMIN-C™
Skin Bleaching &
Correcting Crème
with 20% Vitamin CHydroquinone USP, 4%
RX ONLY
Net Wt. 85 g / 2.99 Oz.

-
INGREDIENTS AND APPEARANCE
ZO MEDICAL MELAMIN-C SKIN BLEACHING AND CORRECTING WITH VITAMIN C HYDROQUINONE
hydroquinone creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42851-034 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROQUINONE (UNII: XV74C1N1AE) (HYDROQUINONE - UNII:XV74C1N1AE) HYDROQUINONE 0.04 g in 1 g Inactive Ingredients Ingredient Name Strength Ascorbic Acid (UNII: PQ6CK8PD0R) Ascorbyl Palmitate (UNII: QN83US2B0N) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Caprylyl Trisiloxane (UNII: Q95M2P1KJL) Cyclomethicone 4 (UNII: CZ227117JE) Dimethicone (UNII: 92RU3N3Y1O) Dimethicone Crosspolymer (450000 MPA.S AT 12% in Cyclopentasiloxane) (UNII: UF7620L1W6) .Alpha.-Tocopherol Acetate, D- (UNII: A7E6112E4N) Diethylene Glycol Monoethyl Ether (UNII: A1A1I8X02B) Sunflower Oil (UNII: 3W1JG795YI) Oleyl Alcohol (UNII: 172F2WN8DV) Plukenetia Volubilis Seed Oil (UNII: 8ED72Z8J1Z) Vitamin A Palmitate (UNII: 1D1K0N0VVC) Rosemary (UNII: IJ67X351P9) Squalane (UNII: GW89575KF9) Tocopherol (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42851-034-85 1 in 1 CARTON 06/01/2016 1 85 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 42851-034-34 1 in 1 CARTON 06/01/2016 2 34 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 06/01/2016 Labeler - ZO Skin Health, Inc. (826468527) Establishment Name Address ID/FEI Business Operations G.S. COSMECEUTICAL USA, INC. 017014734 MANUFACTURE(42851-034)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.